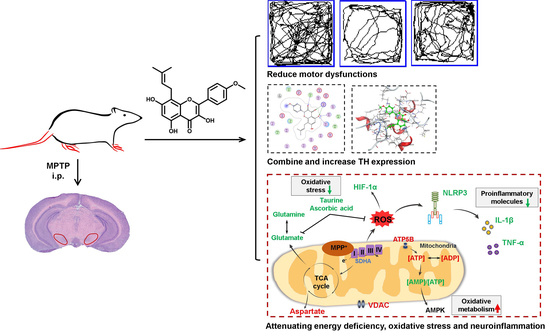
Icaritin Provides Neuroprotection in Parkinson’s Disease by Attenuating Neuroinflammation, Oxidative Stress, and Energy Deficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Drugs
2.2. Animals Care
2.3. MPTP Mouse Model of PD
2.4. Behavioral Assessments
2.4.1. Open Field Test
2.4.2. Rotarod Test
2.4.3. Vertical Grid Test
2.5. Measurement of Serum IL-1β and TNF-α Levels
2.6. Measurement of Serotonin, DA, and the Metabolites in Striatum
2.7. Western Blotting Analysis
2.8. Molecular Docking
2.9. Matrix-Assisted Laser Desorption/Ionisation–Mass Spectrometry Imaging (MALDI-MSI)
2.10. Statistical Analysis
3. Results
3.1. Icaritin Improves Motor Dysfunction and Body Weight in PD Mice
3.2. Icaritin Reduced Dopaminergic Neuronal Damage by Inhibiting NLRP3 Inflammasome Activation
3.3. Icaritin Increases the Level of Antioxidant Molecules and Regulates Glutamate–Glutamine Cycle in the Substantia Nigra, a motor nucleus present in the midbrain
3.4. Icaritin Ameliorates Mitochondrial Function in the Substantia Nigra
3.5. Icaritin Might Interact with Blood–Brain Barrier Related Proteins to Improve PD Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldberg, E.L.; Dixit, V.D. Drivers of age-related inflammation and strategies for healthspan extension. Immunol. Rev. 2015, 265, 63–74. [Google Scholar] [CrossRef]
- Blandini, F.; Armentero, M.T. Animal models of Parkinson’s disease. FEBS J. 2012, 279, 1156–1166. [Google Scholar] [CrossRef] [Green Version]
- Samii, A.; Nutt, J.G.; Ransom, B.R. Parkinson’s disease. Lancet 2004, 363, 1783–1793. [Google Scholar] [CrossRef] [Green Version]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Han, X.; Sun, S.; Sun, Y.; Song, Q.; Zhu, J.; Song, N.; Chen, M.; Sun, T.; Xia, M.; Ding, J.; et al. Small molecule-driven NLRP3 inflammation inhibition via interplay between ubiquitination and autophagy: Implications for Parkinson disease. Autophagy 2019, 15, 1860–1881. [Google Scholar] [CrossRef]
- Youm, Y.H.; Grant, R.W.; McCabe, L.R.; Albarado, D.C.; Nguyen, K.Y.; Ravussin, A.; Pistell, P.; Newman, S.; Carter, R.; Laque, A.; et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013, 18, 519–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, D.R.; Galluzzi, L.; Kroemer, G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 2011, 333, 1109–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burbulla, L.F.; Song, P.; Mazzulli, J.R.; Zampese, E.; Wong, Y.C.; Jeon, S.; Santos, D.P.; Blanz, J.; Obermaier, C.D.; Strojny, C.; et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science 2017, 357, 1255–1261. [Google Scholar] [CrossRef] [Green Version]
- Dawson, T.M.; Dawson, V.L. Molecular pathways of neurodegeneration in Parkinson’s disease. Science 2003, 302, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Rees, K.; Stowe, R.; Patel, S.; Ives, N.; Breen, K.; Clarke, C.E.; Ben-Shlomo, Y. Non-steroidal anti-inflammatory drugs as disease-modifying agents for Parkinson’s disease: Evidence from observational studies. Cochrane Database Syst. Rev. 2011, Cd008454. [Google Scholar] [CrossRef]
- Olanow, C.W.; Rascol, O.; Hauser, R.; Feigin, P.D.; Jankovic, J.; Lang, A.; Tolosa, E. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N. Engl. J. Med. 2009, 361, 1268–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athauda, D.; Foltynie, T. The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat. Rev. Neurol. 2015, 11, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Tatton, N.A.; Kish, S.J. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience 1997, 77, 1037–1048. [Google Scholar] [CrossRef]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007, 2, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xing, B.; Yang, L.; Shi, J.; Zhou, X. Icaritin Attenuates Myocardial Ischemia and Reperfusion Injury Via Anti-Inflammatory and Anti-Oxidative Stress Effects in Rats. Am. J. Chin. Med. 2015, 43, 1083–1097. [Google Scholar] [CrossRef]
- Lai, X.; Ye, Y.; Sun, C.; Huang, X.; Tang, X.; Zeng, X.; Yin, P.; Zeng, Y. Icaritin exhibits anti-inflammatory effects in the mouse peritoneal macrophages and peritonitis model. Int. Immunopharmacol. 2013, 16, 41–49. [Google Scholar] [CrossRef]
- Kang, H.K.; Choi, Y.-H.; Kwon, H.; Lee, S.-B.; Kim, D.-H.; Sung, C.K.; Park, Y.I.; Dong, M.-S. Estrogenic/antiestrogenic activities of a Epimedium koreanum extract and its major components: In vitro and in vivo studies. Food Chem. Toxicol. 2012, 50, 2751–2759. [Google Scholar] [CrossRef]
- Wu, H.; Kim, M.; Han, J. Icariin Metabolism by Human Intestinal Microflora. Molecules 2016, 21, 1158. [Google Scholar] [CrossRef]
- Chen, Y.J.; Zheng, H.Y.; Huang, X.X.; Han, S.X.; Zhang, D.S.; Ni, J.Z.; He, X.Y. Neuroprotective Effects of Icariin on Brain Metabolism, Mitochondrial Functions, and Cognition in Triple-Transgenic Alzheimer’s Disease Mice. CNS Neurosci. Ther. 2016, 22, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.; L’Imperio, V.; Denti, V.; Mazza, M.; Ivanova, M.; Stella, M.; Piga, I.; Chinello, C.; Ajello, E.; Pieruzzi, F.; et al. High Spatial Resolution MALDI-MS Imaging in the Study of Membranous Nephropathy. Proteom. Clin. Appl. 2019, 13, e1800016. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, W.; He, Q.; Xue, J.; Wang, J.; Xiong, C.; Pu, X.; Nie, Z. Mass Spectrometry Imaging of Kidney Tissue Sections of Rat Subjected to Unilateral Ureteral Obstruction. Sci. Rep. 2017, 7, 41954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, S.N.; Birla, H.; Singh, S.S.; Zahra, W.; Patil, R.R.; Jadhav, J.P.; Gedda, M.R.; Singh, S.P. Mucuna pruriens Protects against MPTP Intoxicated Neuroinflammation in Parkinson’s Disease through NF-kappaB/pAKT Signaling Pathways. Front. Aging Neurosci. 2017, 9, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitali, R.; Clarke, S. Improved rotorod performance and hyperactivity in mice deficient in a protein repair methyltransferase. Behav. Brain. Res. 2014, 153, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.T.; Son, H.J.; Choi, J.H.; Ji, I.J.; Hwang, O. Vertical grid test and modified horizontal grid test are sensitive methods for evaluating motor dysfunctions in the MPTP mouse model of Parkinson’s disease. Brain. Res. 2010, 1306, 176–183. [Google Scholar] [CrossRef]
- Friedman, L.K.; Mytilineou, C. Neurochemical and toxic effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridine to rat serotonin neurons in dissociated cell cultures. J. Pharmacol. Exp. Ther. 1990, 253, 892–898. [Google Scholar]
- Zhang, Y.; Wang, W.; Zhang, J. Effects of novel anxiolytic 4-butyl-alpha-agarofuran on levels of monoamine neurotransmitters in rats. Eur. J. Pharmacol. 2004, 504, 39–44. [Google Scholar] [CrossRef]
- MOE. 1010 Sherbooke St. West, Suite #910; H3A 2R7; Chemical Computing Group Inc.: Montreal, QC, Canada, 2019. [Google Scholar]
- Liu, H.; Chen, R.; Wang, J.; Chen, S.; Xiong, C.; Wang, J.; Hou, J.; He, Q.; Zhang, N.; Nie, Z.; et al. 1,5-Diaminonaphthalene hydrochloride assisted laser desorption/ionization mass spectrometry imaging of small molecules in tissues following focal cerebral ischemia. Anal. Chem. 2014, 86, 10114–10121. [Google Scholar] [CrossRef]
- Heinonen, E.H.; Myllyla, V. Safety of selegiline (deprenyl) in the treatment of Parkinson’s disease. Drug. Saf. 1998, 19, 11–22. [Google Scholar] [CrossRef]
- Patel, M.N.; Carroll, R.G.; Galván-Peña, S.; Mills, E.L.; Olden, R.; Triantafilou, M.; Wolf, A.I.; Bryant, C.E.; Triantafilou, K.; Masters, S.L. Inflammasome Priming in Sterile Inflammatory Disease. Trends. Mol. Med. 2017, 23, 165–180. [Google Scholar] [CrossRef]
- Kigerl, K.A.; de Rivero Vaccari, J.P.; Dietrich, W.D.; Popovich, P.G.; Keane, R.W. Pattern recognition receptors and central nervous system repair. Exp. Neurol. 2014, 258, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Menzie, J.; Pan, C.; Prentice, H.; Wu, J.Y. Taurine and central nervous system disorders. Amino Acids 2014, 46, 31–46. [Google Scholar] [CrossRef]
- Fernandez-Calle, P.; Jimenez-Jimenez, F.J.; Molina, J.A.; Cabrera-Valdivia, F.; Vazquez, A.; Garcia Urra, D.; Bermejo, F.; Cruz Matallana, M.; Codoceo, R. Serum levels of ascorbic acid (vitamin C) in patients with Parkinson’s disease. J. Neurol. Sci. 1993, 118, 25–28. [Google Scholar] [CrossRef]
- Kocot, J.; Luchowska-Kocot, D.; Kielczykowska, M.; Musik, I.; Kurzepa, J. Does Vitamin C Influence Neurodegenerative Diseases and Psychiatric Disorders? Nutrients 2019, 9, 659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Zhou, L.; Shimin, Z.; Zhao, Y.; Lin, H.; Zhang, M.; Zhao, S.; Yang, Y.; Ling, Z.; Guan, K.; et al. SIRT3-dependent GOT2 acetylation status affects the malate-aspartate NADH shuttle activity and pancreatic tumor growth. EMBO J. 2015, 34, 1110–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Yang, L.; Xu, Y.W.; Liang, H.; Han, J.; Zhao, R.J.; Cheng, Y. Neuroprotection of hydroxysafflor yellow A in the transient focal ischemia: Inhibition of protein oxidation/nitration, 12/15-lipoxygenase and blood–brain barrier disruption. Brain. Res. 2012, 1473, 227–235. [Google Scholar] [CrossRef]
- Slava, R.; Nathan, A.H.; Sachin, G.; Seliga, A.; Reichenbach, N.L.; Persidsky, Y. Hyperglycemia and advanced glycation end products disrupt BBB and promote occludin and claudin-5 protein secretion on extracellular microvesicles. Sci. Rep. 2020, 10, 7274. [Google Scholar]
- Simona, F.S.; Sara, M.; Yasuteru, S.; Takashi, K.; Maria, A.S. Protective effect of the sphingosine-1 phosphate receptor agonist siponimod on disrupted blood brain barrier function. Biochem. Pharmacol. 2021, 186, 114465. [Google Scholar]
- Choi, J.S.; Park, C.; Jeong, J.W. AMP-activated protein kinase is activated in Parkinson’s disease models mediated by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Biochem. Biophys. Res. Commun. 2010, 391, 147–151. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Liu, L.; Wang, X.; Ding, C.; Tian, Z.; Zhou, R. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 2015, 160, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Lu, M.; Du, R.H.; Qiao, C.; Jiang, C.Y.; Zhang, K.Z.; Ding, J.H.; Hu, G. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol. Neurodegener. 2016, 11, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamik, M.K.; Power, C. Inflammasomes in neurological diseases: Emerging pathogenic and therapeutic concepts. Brain 2017, 140, 2273–2285. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.M.; Ko, H.S.; Dawson, V.L. Genetic animal models of Parkinson’s disease. Neuron 2010, 66, 646–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Lu, X.; Zhang, L.; Wang, L.; Zhang, G.; Yao, J.; Sun, C. Icaritin activates Nrf2/Keap1 signaling to protect neuronal cells from oxidative stress. Chem. Biol. Drug. Des. 2021, 97, 111–120. [Google Scholar] [CrossRef]
- Bagga, P.; Pickup, S.; Crescenzi, R.; Martinez, D.; Borthakur, A.; D’Aquilla, K.; Singh, A.; Verma, G.; Detre, J.A.; Greenberg, J.; et al. In vivo GluCEST MRI: Reproducibility, background contribution and source of glutamate changes in the MPTP model of Parkinson’s disease. Sci. Rep. 2018, 8, 2883. [Google Scholar] [CrossRef] [Green Version]
- Schapira, A.H. Mitochondrial disease. Lancet 2016, 368, 70–82. [Google Scholar] [CrossRef]
- Muqit, M.M.; Gandhi, S.; Wood, N.W. Mitochondria in Parkinson disease: Back in fashion with a little help from genetics. Arch. Neurol. 2006, 63, 649–654. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, S.R.; Chesselet, M.F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog. Neurobiol. 2013, 106–107, 17–32. [Google Scholar] [CrossRef] [Green Version]
- Bueler, H. Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson’s disease. Exp. Neurol. 2009, 218, 235–246. [Google Scholar] [CrossRef]
- Bayliss, J.A.; Lemus, M.B.; Stark, R.; Santos, V.V.; Thompson, A.; Rees, D.J.; Galic, S.; Elsworth, J.D.; Kemp, B.E.; Davies, J.S.; et al. Ghrelin-AMPK Signaling Mediates the Neuroprotective Effects of Calorie Restriction in Parkinson’s Disease. J. Neurosci. 2016, 36, 3049–3063. [Google Scholar] [CrossRef] [Green Version]





| ID | Molecules | Full Name of Target | Source | PDB ID 1 | Docking Score (kcal/mol) | Binding Free Energy (kcal/mol) |
|---|---|---|---|---|---|---|
| 1 | icaritin | tyrosine hydroxylase | Homo | 2XSN | −7.749 | −156.926 |
| 2 | icaritin | voltage-dependent anion channel 1 | Mus | 3EMN | −5.8431 | −130.237 |
| 3 | icaritin | hypoxia-inducible factor-1α | Mus | 4ZPR | −5.3245 | −74.195 |
| 4 | icaritin | ATP synthase subunit 5 beta | Rat | 1mab | −7.082(ADP site) −10.803(ATP site) | −49.804(ADP site) −344.802(ATP site) |
| 5 | icaritin | succinate dehydrogenase subunit A | Homo | 6vax | −7.078 | −159.603 |
| 6 | icaritin | NLR family pyrin domain containing 3 | Homo | 6npy | −8.118 | −134.135 |
| 7 | icaritin | interleukin-1β | Mus | 2mib | −6.217(site 1 in Table S1) | −65.829(site 1 in Table S1) |
| 8 | icaritin | tumor necrosis factor-α | Mus | 2tnf | −7.440(site 1 in Table S2) | −52.203(site 1 in Table S2) |
| 9 | icaritin | 12-lipoxygenase | Homo | 3D3L | −4.83 | −24.365 |
| 10 | icaritin | 15-lipoxygenase | Homo | 1LOX | −8.67 | −44.408 |
| 11 | icaritin | occludin | Homo | 1XAW | −6.94 | −20.161 |
| 12 | icaritin | claudin-5 | Homo | 6OV2 | −6.10 | −32.464 |
| 13 | icaritin | zonula occludens-1 | Homo | 4OEO | −6.85 | −32.049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Liu, X.; Gao, Z.-Y.; Lin, M.; Zhao, X.; Sun, Y.; Pu, X.-P. Icaritin Provides Neuroprotection in Parkinson’s Disease by Attenuating Neuroinflammation, Oxidative Stress, and Energy Deficiency. Antioxidants 2021, 10, 529. https://doi.org/10.3390/antiox10040529
Wu H, Liu X, Gao Z-Y, Lin M, Zhao X, Sun Y, Pu X-P. Icaritin Provides Neuroprotection in Parkinson’s Disease by Attenuating Neuroinflammation, Oxidative Stress, and Energy Deficiency. Antioxidants. 2021; 10(4):529. https://doi.org/10.3390/antiox10040529
Chicago/Turabian StyleWu, Hao, Xi Liu, Ze-Yu Gao, Ming Lin, Xin Zhao, Yi Sun, and Xiao-Ping Pu. 2021. "Icaritin Provides Neuroprotection in Parkinson’s Disease by Attenuating Neuroinflammation, Oxidative Stress, and Energy Deficiency" Antioxidants 10, no. 4: 529. https://doi.org/10.3390/antiox10040529
APA StyleWu, H., Liu, X., Gao, Z. -Y., Lin, M., Zhao, X., Sun, Y., & Pu, X. -P. (2021). Icaritin Provides Neuroprotection in Parkinson’s Disease by Attenuating Neuroinflammation, Oxidative Stress, and Energy Deficiency. Antioxidants, 10(4), 529. https://doi.org/10.3390/antiox10040529