Risk Assessment of Oxidative Stress Induced by Metal Ions Released from Fixed Orthodontic Appliances during Treatment and Indications for Supportive Antioxidant Therapy: A Narrative Review
Abstract
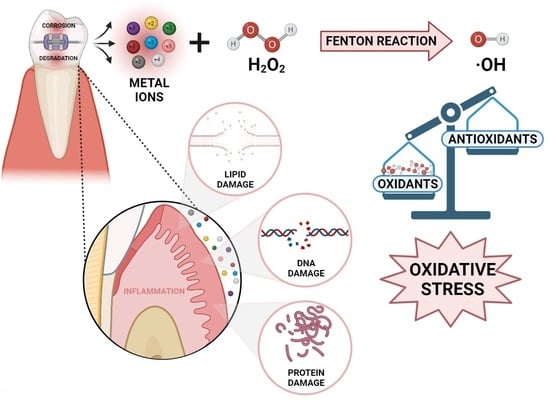
:1. Transition Metal Ions, Fenton-like Chemistry and Oxidative Stress
2. Fixed Orthodontic Appliances
3. Metal Ions Release during Treatment with Fixed Orthodontic Appliances
4. Cytotoxicity
5. Oxidative Stress and Oxidative Damage during the Treatment with Fixed Orthodontic Appliances
6. Risk Assessment
| Source of Metal Ions | NOAEL | LOAEL | Concentration (Dose) | Study Type | Model Organism | Ref. |
| Fe | ||||||
| FeSO4 | 10 μM | 250 μM | 10–1000 μM | acute toxicity | human leukocytes | [72] |
| 50 μM | 100 μM | 0, 10, 25, 50, 100 μM | acute toxicity | microglia cells | [73] | |
| FeCl3·6H2O | 3.2 μM | 11.1 μM | 3.2, 11.1, 111, 1111 μM | chronic toxicity | rats | [74] * |
| 100 µM | 200 µM | 0–1400 μM | acute toxicity | BALB/3T3, HepG2 cells | [75] | |
| iron sucrose injection | 1790 µM | 3580 µM | 360, 890, 1790, 3580, 8950 µM | acute toxicity | hemodialysis patients | [76] * |
| Ni | ||||||
| NiCl2 | 27.2 mg/kg | 54.4 mg/kg | 3.4, 6.8, 13.6, 27.2, 54.4, 108.8 mg/kg | acute toxicity | mice | [77] |
| 100 µM | 250 µM | 0, 100, 250, 500, 600, 750, 1000 µM | acute toxicity | human HeLa cells | [78] | |
| 10 µM | 100 µM | 0–10,000 µM | acute toxicity | human keratinocytes | [79] | |
| NiCl2·6H2O | 100 µM | 200 µM | 0–1400 μM | acute toxicity | BALB/3T3 cells | [75] |
| NiSO4 | 1.25 mg/kg/day | 2.5 mg/kg/day | 1.25, 2.5, 5 mg/kg/day | sub-acute toxicity | rats | [80] |
| Ni(NO3)2 | 150 μM | 300 μM | 0, 30, 75, 150, 300, 600 μM | acute toxicity | chromatin from rat liver cells | [81] |
| Cr | ||||||
| K2Cr2O7 | 1 µM | 10 µM | 0–1000 µM | acute toxicity | human keratinocytes | [79] |
| 50 µM | 400 µM | 50, 100, 200, 400, 600, 1000 μM | acute toxicity | lymphocytes | [82] | |
| CrCl3 | 400 µM | 600 µM | 50, 100, 200, 400, 600, 1000 μM | acute toxicity | lymphocytes | [82] |
| CrCl3·6H2O | 100 µM | 200 µM | 0–1400 μM | acute toxicity | BALB/3T3 cells | [75] |
| Mo | ||||||
| MoO3 | 100 µM | 200 µM | 0–1400 μM | acute toxicity | BALB/3T3, HepG2 cells | [75] |
| Na2MoO4 | 60.7 µM | 121.4 µM | 0, 60.7, 121.4, 242.7, 485.4, 970.9 µM | Sub- acute toxicity | mice | [83] * |
| MoCl5 | 100 µM | 500 µM | 50, 100, 500, 1000, 5000 µM | acute toxicity | human CD4þ T lymphocytes | [58] * |
| Ti | ||||||
| Ti particles | 13 µM | 26 µM | 13, 26, 52, 104, 209 µM | acute toxicity | Osteoblasts MC3T3-E1 | [84] * |
| Co | ||||||
| CoCl2 | 50 µM | 100 µM | 50, 100, 500, 1000, 5000 µM | acute toxicity | human CD4þ T lymphocytes | [58] * |
| CoCl2 | / | 1 µM | 1–100 μM | acute toxicity | Balb/3T3 mouse fibroblasts | [85] |
| Metal | Threshold | References | |
|---|---|---|---|
| Men | Women | ||
| Iron | RDA * = 8 mg/day | RDA = 18 mg/dayRDA = 8 mg/day (postmenopause) | [90] |
| UL * = 45 mg/day | UL = 45 mg/day | [90] | |
| RDA = 17 mg/day | RDA = 17 mg/day | [91] | |
| AI * = 11 mg/day | AI = 16 mg/dayAI = 11 mg/day (postmenopause) | [92] | |
| RDA = 10 mg/day | RDA = 10–15 mg/day | [93] | |
| DNEL * = 710 µg/kg bw/day (chronic exposure) | DNEL * = 710 µg/kg bw/day (chronic exposure) | [94] | |
| Nickel | UL = 1.0 mg/day | UL = 1.0 mg/day | [95] |
| GL = 0.26 mg/day | GL = 0.26 mg/day | [91] | |
| DNEL = 11 µg/kg bw/day (chronic exposure) | DNEL = 11 µg/kg bw/day (chronic exposure) | [96] | |
| Chromium | AI = 35 μg/day | AI = 25 μg/day | [97] |
| RDA = 30–100 µg/day | RDA = 30–100 µg/day | [93] | |
| GL = 10 mg/day | GL = 10 mg/day | [91] | |
| Titanium | DNEL = 350 mg/kg bw/day (chronic exposure) | DNEL = 350 mg/kg bw/day (chronic exposure) | [98] |
| Molybdenum | AI = 45 μg/day | AI = 45 μg/day | [99] |
| RDA = 65 μg/day | RDA = 65 μg/day | [100] | |
| RDA = 50–100 μg/day | RDA = 50–100 μg/day | [93] | |
| DNEL = 3.4 mg/kg bw/day | DNEL = 3.4 mg/kg bw/day | [101] | |
| Cobalt | GL = 1.4 mg/day | GL = 1.4 mg/day | [91] |
| DNEL = 29.8 µg/kg bw/day (chronic exposure) | DNEL = 29.8 µg/kg bw/day (chronic exposure) | [102] | |
| Metal Ion Release from Orthodontic Appliances | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fe | Ni | Cr | Ti | Mo | Co | Alloy Type | Time | Ref. |
| 310 ± 123.1 ppb | 1292 ± 437.5 ppb | 0 ppb | / | / | 4 ± 5.477 ppb | SS wire + SS brackets + SS band | 90 days | [106] |
| 450 ± 222.8 ppb | 1864 ± 600.2 ppb | 18 ± 34.9 ppb | / | / | 10 ± 7.071 ppb | NiTi wire + SS brackets + SS band | ||
| 614 ± 531.7 ppb | 2466 ± 867.8 ppb | 30 ± 51.9 ppb | / | / | 12 ± 4.47 ppb | Co-NiTi wire + SS brackets + SS band | ||
| 534 ± 558 ppb | 2132 ± 1143 ppb | 20 ± 44.72 ppb | / | / | 12 ± 4.472 ppb | Elgiloy wire + SS brackets + SS band | ||
| 2382 ppb | 573 ppb | 101 ppb | / | / | / | SS wires, SS brackets, SS bands, SS metal ligatures | 30 days | [34] |
| 1107 ppb | 15 ppb | 8 ppb | / | / | / | control | ||
| / | 18.5 ± 13.1 ppb | 2.6 ± 1.6 ppb | / | / | / | SS brackets and bands; SS + NiTi archwires | 16 ± 2 months | [29] |
| / | 11.9 ± 11.4 ppb | 2.2 ± 1.8 ppb | / | / | / | control | ||
| 10.78 ± 17.66 µg | 28.33 ± 29.19 µg | 12.66 ± 4.61 µg | / | 133.33 ± 57.7 µg | / | NiTi archwire + Dentaurum brackets | 28 days | [107] |
| 21.33 ± 8.73 µg | 17.66 ± 11/59 µg | <10 ± 0 µg | / | 110 ± 17.32 µg | / | NiTi archwire + American Ortho brackets | ||
| 14.33 ± 5.33 µg | 76.66 ± 62.52 µg | 16.66 ± 11.54 µg | / | <130 ± 0 µg | / | NiTi archwire + Shinye brackets | ||
| <10 ± 0 µg | 22 ± 7.54 µg | 30 ± 0 µg | / | 120 ± 17.32 µg | / | NiTi archwire + ORJ brackets | ||
| 16 ± 5.29 µg | 37 ± 20.042 µg | 26 ± 6.92 µg | / | 110 ± 17.32 µg | / | SS archwire + Dentaurum brackets | ||
| 11 ± 1.73 µg | 45 ± 31 µg | 26 ± 6.92 µg | / | 0 ± <100 µg | / | SS archwire + American Ortho brackets | ||
| <10 ± 0 µg | 293.2 ± 365.6 µg | 15.33 ± 4.61 µg | / | 0 ± <100 µg | / | SS archwire + Shinye brackets | ||
| <10 ± 0 µg | 44.66 ± 35.50 µg | 15.33 ± 4.61 µg | / | 110 ± 17.32 µg | / | SS archwire + ORJ brackets | ||
| 67.7 ± 9.6 ppb | 10 ± 6.3 ppb | 4.5 ± 0.5 ppb | 2 ± 0.6 ppb | / | / | NiTi New brackets + new archwire | 45 days | [41] |
| 64.3 ± 8 ppb | 15 ± 3.3 ppb | 4 ± 0.7 ppb | 2 ± 0.7 ppb | / | / | NiTi Recycled brackets + new archwire | ||
| 115 ± 11.9 ppb | 20 ± 6.5 ppb | 7.3 ± 0.6 ppb | 3.5 ± 1.3 ppb | / | / | NiTi New brackets + recycled archwire | ||
| 140 ± 9.4 ppb | 20 ± 3.3 ppb | 7.5 ± 0,6 ppb | 4 ± 0.7 ppb | / | / | NiTi Recycled brackets + recycled archwire | ||
| / | 2 ppb | / | / | / | / | NiTi archwire | 28 days | [108] |
| / | / | / | / | / | / | TMO archwire | ||
| 289 ppb | 6 ppb | 11 ppb | / | / | / | SS archwire | ||
| / | 4 ppb | 3 ppb | / | / | / | Cu-NiTi archwire | ||
| / | 67 ± 10.8 ppb | 30.8 ± 4.3 ppb | / | / | / | SS brackets, bands NiTi & SS archwires | 1.5 years | [31] |
| / | 5.02 ± 0.001 ppb | 1.27 ± 0.09 ppb | / | / | / | control | ||
| / | 0.93 ± 0.04 μg | / | / | / | / | NiTi archwires | 21 days | [35] |
| / | 0.66 ± 0.02 μg | / | / | / | / | SS archwires | ||
| / | 0.67 ± 0.02 μg | / | / | / | / | CuNiTi archwires | ||
| / | 0.77 ± 0.05 μg | / | / | / | / | Ion implanted NiTi archwires | ||
| / | 125 ± 22 μg | 112 ± 18 μg | / | / | / | SS ¼ archwire + bands + brackets | 12 days | [40] |
| 96.06 ± 57.4 ppb/day | 41.66 ± 33.99 ppb/day | 33.43 ± 24.05 ppb/day | 0 ± 0 | / | / | SS archwire | 1st day | [38] |
| 25.55 ± 10.00 ppb/day | 10.21 ± 2.68 ppb/day | 3.83 ± 1.93 ppb/day | 0 ± 0 | / | / | 6th day | ||
| 11.08 ± 5.89 ppb/day | 5.28 ± 1.87 ppb/day | 1.43 ± 0.69 ppb/day | 0 ± 0 | / | / | 7th day | ||
| 5.62 ± 1.47 ppb/day | 3.84 ± 0.86 ppb/day | 0.70 ± 0.10 ppb/day | 0 ± 0 | / | / | 14th day | ||
| 38.47 ± 15.67 ppb/day | 11.77 ± 2.84 ppb/day | 10.49 ± 3.90 ppb/day | 0.14 ± 0.04 ppb/day | / | / | NiTi archwire | 1st day | [38] |
| 26.93 ± 5.44 ppb/day | 10.83 ± 3.49 ppb/day | 3.30 ± 0.95 ppb/day | 0.01 ± 0 ppb/day | / | / | 6th day | ||
| 13.07 ± 4.01 ppb/day | 6.13 ± 1.39 ppb/day | 1.76 ± 0.34 ppb/day | 0.01 ± 0.01 ppb/day | / | / | 7th day | ||
| 6.81 ± 1.70 ppb/day | 3.38 ± 1.67 ppb/day | 1.06 ± 0.21 ppb/day | 0.01 ± 0 ppb/day | / | / | 14th day | ||
| 21.18 ± 6,43 ppb/day | 7.12 ± 1.33 ppb/day | 4.39 ± 1.99 ppb/day | 0.12 ± 0.02 ppb/day | / | / | Termo NiTi archwire | 1st day | [38] |
| 19.11 ± 6.28 ppb/day | 7.26 ± 1.10 ppb/day | 2.10 ± 0.84 ppb/day | 0.01 ± 0 ppb/day | / | / | 6th day | ||
| 8.79 ± 3.79 ppb/day | 5.06 ± 1.57 ppb/day | 1.23 ± 0.80 ppb/day | 0.01 ± 0 ppb/day | / | / | 7th day | ||
| 2.50 ± 0.59 ppb/day | 2.33 ± 0.77 ppb/day | 0.40 ± 0.15 ppb/day | 0 ± 0 | / | / | 14th day | ||
| 42.33 ± 27.06 ppb | 0.00 ± 0.00 ppb | 0.36 ± 0.33 ppb | 6.64 ± 2.00 ppb | 0.80 ± 0.27 ppb | 0.00 ± 0.00 ppb | control | 30 days | [25] |
| 520.0 ± 210.1 ppb | 416.9 ± 133.5 ppb | 8.97 ± 6.73 ppb | 9.03 ± 2.08 ppb | 3.11 ± 1.47 ppb | 1.38 ± 1.25 ppb | SS brackets + SS bands | ||
| 49.67 ± 29.29 ppb | 0.00 ± 0.00 ppb | 0.06 ± 0.05 ppb | 5.35 ± 3.96 ppb | 0.11 ± 0.07 ppb | 0.03 ± 0.01 ppb | Ti brackets + Ti bands | ||
| 179.49 ± 99.1 ppb | 5.79 ± 2.07 ppb | 0.91 ± 0.28 ppb | 3.79 ± 3.11 ppb | 0.60 ± 0.21 ppb | 0.00 ± 0.00 ppb | Ni-free brackets + bands | ||
7. Indications for Antioxidant Therapy: Pros and Cons
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, 31–38. [Google Scholar] [CrossRef]
- Deguillaume, L.; Leriche, M.; Chaumerliac, N. Impact of radical versus non-radical pathway in the Fenton chemistry on the iron redox cycle in clouds. Chemosphere 2005, 60, 718–724. [Google Scholar] [CrossRef]
- Poljsak, B. Strategies for reducing or preventing the generation of oxidative stress. Oxidative Med. Cell. Longev. 2011, 2011, 194586. [Google Scholar] [CrossRef] [Green Version]
- Nuran Ercal, B.S.P.; Hande Gurer-Orhan, B.S.P.; Nukhet Aykin-Burns, B.S.P.; Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress. Part I: Mechanisms involved in metal induced oxidative damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, B.; Jamnik, P.; Raspor, P.; Pesti, M. Oxidation-Antioxidation-Reduction Processes in the Cell: Impacts of Environmental Pollution. In Encyclopedia of Environmental Health; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 300–306. ISBN 9780444522726. [Google Scholar]
- Poljsak, B.; Milisav, I. The neglected significance of “antioxidative stress”. Oxidative Med. Cell. Longev. 2012, 2012, 480895. [Google Scholar] [CrossRef] [Green Version]
- Kell, D.B. Iron behaving badly: Inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med. Genom. 2009, 2, 2. [Google Scholar] [CrossRef]
- Kanti Das, T.; Wati, M.R.; Fatima-Shad, K. Oxidative Stress Gated by Fenton and Haber Weiss Reactions and Its Association With Alzheimer’s Disease. Arch. Neurosci. 2014, 2, e60038. [Google Scholar] [CrossRef] [Green Version]
- Torreilles, J.; Guerin, M.C.; Slaoui-Hasnaoui, A. Nickel (II) complexes of histidyl-peptides as fenton-reaction catalysts. Free Radic. Res. 1990, 11, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Biologically relevant metal ion-dependent hydroxyl radical generation An update. FEBS Lett. 1992, 307, 108–112. [Google Scholar] [CrossRef] [Green Version]
- Sugden, K.D.; Geer, R.D.; Rogers, S.J. Oxygen Radical-Mediated DNA Damage by Redox-Active Cr(III) Complexes. Biochemistry 1992, 31, 11626–11631. [Google Scholar] [CrossRef]
- Glaser, V.; Leipnitz, G.; Straliotto, M.R.; Oliveira, J.; dos Santos, V.V.; Wannmacher, C.M.D.; de Bem, A.F.; Rocha, J.B.T.; Farina, M.; Latini, A. Oxidative stress-mediated inhibition of brain creatine kinase activity by methylmercury. Neurotoxicology 2010, 31, 454–460. [Google Scholar] [CrossRef]
- Sifakakis, I.; Eliades, T. Adverse reactions to orthodontic materials. Aust. Dent. J. 2017, 62, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucchese, A.; Carinci, F.; Brunelli, G.; Monguzzi, R. An in vitro study of resistance to corrosion in brazed and laser welded orthodontic appliances. Eur. J. Inflamm. 2011, 9, 67–72. [Google Scholar]
- Bandeira, A.M.; Ferreira Martinez, E.; Dias Demasi, A.P. Evaluation of toxicity and response to oxidative stress generated by orthodontic bands in human gingival fibroblasts. Angle Orthod. 2020, 90, 285–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, L.F. The straight-wire appliance. Br. J. Orthod. 1979, 6, 125–143. [Google Scholar] [CrossRef]
- Wichelhaus, A.; Geserick, M.; Hibst, R.; Sander, F.G. The effect of surface treatment and clinical use on friction in NiTi orthodontic wires. Dent. Mater. 2005, 21, 938–945. [Google Scholar] [CrossRef]
- Eliades, T.; Bourauel, C. Intraoral aging of orthodontic materials: The picture we miss and its clinical relevance. Am. J. Orthod. Dentofac. Orthop. 2005, 127, 403–412. [Google Scholar] [CrossRef]
- Eliades, T.; Athanasiou, A.E. In Vivo Aging of Orthodontic Alloys: Implications for Corrosion Potential, Nickel Release, and Biocompatibility. Angle Orthod. 2002, 72, 222–237. [Google Scholar]
- Cortizo, M.C.; De Mele, M.F.L.; Cortizo, A.M. Metallic dental material biocompatibility in osteoblastlike cells: Correlation with metal ion release. Biol. Trace Elem. Res. 2004, 100, 151–168. [Google Scholar] [CrossRef]
- Kao, C.T.; Ding, S.J.; Min, Y.; Hsu, T.C.; Chou, M.Y.; Huang, T.H. The cytotoxicity of orthodontic metal bracket immersion media. Eur. J. Orthod. 2007, 29, 198–203. [Google Scholar] [CrossRef]
- Drescher, D.; Bourauel, C.; Schumacher, H.A. Frictional forces between bracket and arch wire. Am. J. Orthod. Dentofac. Orthop. 1989. [Google Scholar] [CrossRef]
- Landolt, D.; Mischler, S.; Stemp, M. Electrochemical methods in tribocorrosion: A critical appraisal. Electrochim. Acta 2001, 46, 3913–3929. [Google Scholar] [CrossRef]
- Močnik, P.; Kosec, T.; Kovač, J.; Bizjak, M. The effect of pH, fluoride and tribocorrosion on the surface properties of dental archwires. Mater. Sci. Eng. C 2017, 78, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.J.; Fernández, E.; Vicente, A.; Calvo, J.L.; Ortiz, C. Metallic ions released from stainless steel, nickel-free, and titanium orthodontic alloys: Toxicity and DNA damage. Am. J. Orthod. Dentofac. Orthop. 2011, 140, 115–122. [Google Scholar] [CrossRef]
- Keinan, D.; Mass, E.; Zilberman, U. Absorption of Nickel, Chromium, and Iron by the Root Surface of Primary Molars Covered with Stainless Steel Crowns. Int. J. Dent. 2010, 2010, 326124. [Google Scholar] [CrossRef] [Green Version]
- Moresca, R. Orthodontic treatment time: Can it be shortened? Dent. Press J. Orthod. 2018, 23, 90–105. [Google Scholar] [CrossRef] [Green Version]
- Wataha, J.C. Biocompatibility of dental casting alloys: A review. J. Prosthet. Dent. 2000, 83, 223–234. [Google Scholar] [CrossRef]
- Amini, F.; Jafari, A.; Amini, P.; Sepasi, S. Metal ion release from fixed orthodontic appliances—An in vivo study. Eur. J. Orthod. 2012, 34, 126–130. [Google Scholar] [CrossRef] [Green Version]
- Kovac, V.; Poljsak, B.; Perinetti, G.; Primozic, J.; Reis, F.S. Systemic Level of Oxidative Stress during Orthodontic Treatment with Fixed Appliances. Biomed. Res. Int. 2019, 2019, 5063565. [Google Scholar] [CrossRef]
- Quadras, D.; Nayak, U.; Kumari, N.; Priyadarshini, H.; Gowda, S.; Fernandes, B. In vivo study on the release of nickel, chromium, and zinc in saliva and serum from patients treated with fixed orthodontic appliances. Dent. Res. J. 2019, 16, 209–215. [Google Scholar] [CrossRef]
- Kovač, V.; Poljšak, B.; Primožič, J.; Jamnik, P. Are metal ions that make up orthodontic alloys cytotoxic, and do they induce oxidative stress in a yeast cell model? Int. J. Mol. Sci. 2020, 21, 7993. [Google Scholar] [CrossRef]
- Greenwald, A.S. Biological Performance of Materials: Fundamentals of Biocompatibility, 3rd ed.; Taylor Francis Group: Abingdon, UK, 2001; Volume 83. [Google Scholar]
- Mikulewicz, M.; Chojnacka, K.; Woźniak, B.; Downarowicz, P. Release of metal ions from orthodontic appliances: An in vitro study. Biol. Trace Elem. Res. 2012, 146, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Charles, A.; Gangurde, P.; Jacob, S.; Jatol-Tekade, S.; Senkutvan, R.; Vadgaonkar, V. Evaluation of nickel ion release from various orthodontic arch wires: An in vitro study. J. Int. Soc. Prev. Community Dent. 2014, 4, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, H.D.; Ajith, S.D.; Goel, P. Nickel release from stainless steel and nickel titanium archwires—An in vitro study. J. Oral Biol. Craniofacial Res. 2016, 6, 213–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, C.J.; Shin, J.S.; Cha, J.Y. Metal release from simulated fixed orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 2001, 120, 383–391. [Google Scholar] [CrossRef]
- Kuhta, M.; Pavlin, D.; Slaj, M.M.; Varga, S.; Lapter-Varga, M.; Slaj, M.M. Type of archwire and level of acidity: Effects on the release of metal ions from orthodontic appliances. Angle Orthod. 2009, 79, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, R.D.; Bishara, S.E.; Quinn, J.K. Biodegradation of orthodontic appliances. Part I. Biodegradation of nickel and chromium in vitro. Am. J. Orthod. Dentofac. Orthop. 1993, 103, 8–14. [Google Scholar] [CrossRef]
- Park, H.Y.; Shearer, T.R. In vitro release of nickel and chromium from simulated orthodontic appliances. Am. J. Orthod. 1983, 84, 156–159. [Google Scholar] [CrossRef]
- Gürsoy, S.; Acar, A.G.; Şeşen, Ç. Comparison of Metal Release from New and Recycled Bracket-Archwire Combinations. Angle Orthod. 2005, 75, 92–94. [Google Scholar]
- Hussain, S.; Asshaari, A.; Osman, B.; AL-Bayaty, F. In Vitro-Evaluation of Biodegradation of Different Metallic Orthodontic Brackets. J. Int. Dent. Med. Res. 2017, 7, 76–83. [Google Scholar]
- He, L.; Cui, Y.; Zhang, C. Effect of Protein and Mechanical Strain on the Corrosion Resistance and Cytotoxicity of the Orthodontic Composite Arch Wire. ACS Omega 2020, 5, 8992–8998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaskar, V.; Subba Reddy, V. Biodegradation of nickel and chromium from space maintainers: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2010, 28, 6. [Google Scholar] [CrossRef]
- Mikulewicz, M.; Chojnacka, K. Trace Metal release from orthodontic appliances by in vivo studies: A systematic literature review. Biol. Trace Elem. Res. 2010, 137, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Ryhä, J.; Nen, R.; Niemi, E.; Serlo, W.; Niemelä, E.N.; Sandvik, P.; Pernu, H.; Salo, T. Biocompatibility of nickel-titanium shape memory metal and its corrosion behavior in human cell cultures. J. Biomed. Mater. Res. 1997, 35, 451–457. [Google Scholar]
- Wever, D.J.; Veldhuizen, A.G.; Sanders, M.M.; Schakenraad, J.M.; Van Horn, J.R. Cytotoxic, allergic and genotoxic activity of a nickel-titanium alloy. Biomaterials 1997, 18, 1115–1120. [Google Scholar] [CrossRef]
- Rose, E.C.; Jonas, I.E.; Kappert, H.F. In vitro investigation into the biological assessment of orthodontic wires. J. Orofac. Orthop. 1998, 59, 253–264. [Google Scholar] [CrossRef]
- Es-Souni, M.; Fischer-Brandies, H.; Es-Souni, M. In-vitro-bioverträglichkeit von Elgiloy®, einer kobalt-basislegierung, im vergleich zu zwei titanlegierungen. J. Orofac. Orthop. 2003, 64, 16–26. [Google Scholar] [CrossRef]
- Shih, C.-C.; Lin, S.-J.; Chen, Y.-L.; Su, Y.-Y.; Lai, S.-T.; Wu, G.J.; Kwok, C.-F.; Chung, K.-H. The cytotoxicity of corrosion products of nitinol stent wire on cultured smooth muscle cells. J. Biomed. Mater. Res. 2000, 52, 395–403. [Google Scholar] [CrossRef]
- Shih, C.-C.; Shih, C.-M.; Chen, Y.-L.; Su, Y.-Y.; Shih, J.-S.; Kwok, C.-F.; Lin, S.-J. Growth inhibition of cultured smooth muscle cells by corrosion products of 316 L stainless steel wire. J. Biomed. Mater. Res. 2001, 112, 200–207. [Google Scholar] [CrossRef]
- David, A.; Lobner, D. In vitro cytotoxicity of orthodontic archwires in cortical cell cultures. Eur. J. Orthod. 2004, 26, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Spalj, S.; Mlacovic Zrinski, M.; Tudor Spalj, V.; Ivankovic Buljan, Z. In-vitro assessment of oxidative stress generated by orthodontic archwires. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 583–589. [Google Scholar] [CrossRef]
- Yang, H.C.; Pon, L.A. Toxicity of metal ions used in dental alloys: A study in the yeast Saccharomyces cerevisiae. Drug Chem. Toxicol. 2003, 26, 75–85. [Google Scholar] [CrossRef]
- Limberger, K.M.; Westphalen, G.H.; Menezes, L.M.; Medina-Silva, R. Cytotoxicity of orthodontic materials assessed by survival tests in Saccharomyces cerevisiae. Dent. Mater. 2011, 27, e81–e86. [Google Scholar] [CrossRef]
- Buljan, Z.I.; Ribaric, S.P.; Abram, M.; Ivankovic, A.; Spalj, S. In vitro oxidative stress induced by conventional and self-ligating brackets. Angle Orthod. 2012, 82, 340–345. [Google Scholar] [CrossRef] [Green Version]
- Hafez, H.S.; Selim, E.M.N.; Kamel Eid, F.H.; Tawfik, W.A.; Al-Ashkar, E.A.; Mostafa, Y.A. Cytotoxicity, genotoxicity, and metal release in patients with fixed orthodontic appliances: A longitudinal in-vivo study. Am. J. Orthod. Dentofac. Orthop. 2011, 140, 298–308. [Google Scholar] [CrossRef]
- Caicedo, M.; Jacobs, J.J.; Reddy, A.; Hallab, N.J. Analysis of metal ion-induced DNA damage, apoptosis, and necrosis in human (Jurkat) T-cells demonstrates Ni2+ and V3+ are more toxic than other metals: Al3+, Be2+, Co2+, Cr3+, Cu2+, Fe3+, Mo5+, Nb5+, Zr2+. J. Biomed. Mater. Res. Part A 2008, 86, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Ortega, E.; Jos, A.; Cameán, A.M.; Pato-Mourelo, J.; Segura-Egea, J.J. In vitro evaluation of cytotoxicity and genotoxicity of a commercial titanium alloy for dental implantology. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2010, 702, 17–23. [Google Scholar] [CrossRef]
- El Medawar, L.; Rocher, P.; Hornez, J.C.; Traisnel, M.; Breme, J.; Hildebrand, H.F. Electrochemical and cytocompatibility assessment of NiTiNOL memory shape alloy for orthodontic use. In Proceedings of the Biomolecular Engineering; Elsevier: Amsterdam, The Netherlands, 2002; Volume 19, pp. 153–160. [Google Scholar]
- Issa, Y.; Brunton, P.; Waters, C.M.; Watts, D.C. Cytotoxicity of metal ions to human oligodendroglial cells and human gingival fibroblasts assessed by mitochondrial dehydrogenase activity. Dent. Mater. 2008, 24, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Buczko, P.; Knaś, M.; Grycz, M.; Szarmach, I.; Zalewska, A. Orthodontic treatment modifies the oxidant–antioxidant balance in saliva of clinically healthy subjects. Adv. Med. Sci. 2017, 62, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Olteanu, C.; Muresan, A.; Daicoviciu, D.; Tarmure, V.; Olteanu, I.; Irene, K.M.L.W. Variations of some saliva markers of the oxidative stress in patients with orthodontic appliances. Fiziologia 2009, 19, 27–29. [Google Scholar]
- Atuǧ Özcan, S.S.; Ceylan, I.; Özcan, E.; Kurt, N.; Daǧsuyu, I.M.; Çanakçi, C.F. Evaluation of oxidative stress biomarkers in patients with fixed orthodontic appliances. Dis. Markers 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Portelli, M.; Militi, A.; Cervino, G.; Lauritano, F.; Sambataro, S.; Mainardi, A.; Nucera, R. Oxidative Stress Evaluation in Patients Treated with Orthodontic Self-ligating Multibracket Appliances: An Case-Control Study. Open Dent. J. 2017, 11, 257–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrugia, G.; Balzan, R.; Madeo, F.; Breitenbach, M. Oxidative Stress and Programmed Cell Death in Yeast; Frontiers Media SA: Lausanne, Switzerland, 2012; pp. 1–21. [Google Scholar]
- Gonçalves, T.S.; de Menezes, L.M.; Trindade, C.; Machado, M. da S.; Thomas, P.; Fenech, M.; Henriques, J.A.P. Cytotoxicity and genotoxicity of orthodontic bands with or without silver soldered joints. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 762, 1–8. [Google Scholar] [CrossRef]
- Rincic Mlinaric, M.; Durgo, K.; Katic, V.; Spalj, S. Cytotoxicity and oxidative stress induced by nickel and titanium ions from dental alloys on cells of gastrointestinal tract. Toxicol. Appl. Pharmacol. 2019, 383. [Google Scholar] [CrossRef] [PubMed]
- Terpilowska, S.; Siwicki, A.K. Interactions between chromium(III) and iron(III), molybdenum(III) or nickel(II): Cytotoxicity, genotoxicity and mutagenicity studies. Chemosphere 2018, 201, 780–789. [Google Scholar] [CrossRef] [PubMed]
- The International Programme on Chemical Safety. Principles for the Safety Assessment of Food Additives and Contaminants in Food; WHO: Geneva, Switzerland, 1987. [Google Scholar]
- Faustman, E.M.; Omenn, B.S. Risk assessment. In Casarett & Doull’s Toxicology: The Basic Science of Poisons; Klaassen, C.D., Ed.; McGraw: New York, NY, USA, 2001; pp. 92–94. [Google Scholar]
- Park, J.H.; Park, E. Influence of iron-overload on DNA damage and its repair in human leukocytes in vitro. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011, 718, 56–61. [Google Scholar] [CrossRef]
- Yauger, Y.J.; Bermudez, S.; Moritz, K.E.; Glaser, E.; Stoica, B.; Byrnes, K.R. Iron accentuated reactive oxygen species release by NADPH oxidase in activated microglia contributes to oxidative stress in vitro. J. Neuroinflamm. 2019, 16, 1–15. [Google Scholar] [CrossRef]
- Ceylan, H.; Budak, H.; Kocpinar, E.F.; Baltaci, N.G.; Erdogan, O. Examining the link between dose-dependent dietary iron intake and Alzheimer’s disease through oxidative stress in the rat cortex. J. Trace Elem. Med. Biol. 2019, 56, 198–206. [Google Scholar] [CrossRef]
- Terpilowska, S.; Siwicki, A.K. Cell cycle and transmembrane mitochondrial potential analysis after treatment with chromium(iii), iron(iii), molybdenum(iii) or nickel(ii) and their mixtures. Toxicol. Res. 2019, 8, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Kuo, K.L.; Hung, S.C.; Wei, Y.H.; Tarng, D.C. Intravenous iron exacerbates oxidative DNA damage in peripheral blood lymphocytes in chronic hemodialysis patients. J. Am. Soc. Nephrol. 2008, 19, 1817–1826. [Google Scholar] [CrossRef] [Green Version]
- Danadevi, K.; Rozati, R.; Banu, B.S.; Grover, P. In vivo genotoxic effect of nickel chloride in mice leukocytes using comet assay. Food Chem. Toxicol. 2004, 42, 751–757. [Google Scholar] [CrossRef]
- Dally, H.; Hartwig, A. Induction and repair inhibition of oxidative DNA damage by nickel(II) and cadmium(II) in mammalian cells. Carcinogenesis 1997, 18, 1021–1026. [Google Scholar] [CrossRef] [Green Version]
- Curtis, A.; Morton, J.; Balafa, C.; MacNeil, S.; Gawkrodger, D.J.; Warren, N.D.; Evans, G.S. The effects of nickel and chromium on human keratinocytes: Differences in viability, cell associated metal and IL-1α release. Toxicol. Vitr. 2007, 21, 809–819. [Google Scholar] [CrossRef]
- Su, L.; Deng, Y.; Zhang, Y.; Li, C.; Zhang, R.; Sun, Y.; Zhang, K.; Li, J.; Yao, S. Protective effects of grape seed procyanidin extract against nickel sulfate-induced apoptosis and oxidative stress in rat testes. Toxicol. Mech. Methods 2011, 21, 487–494. [Google Scholar] [CrossRef]
- Rabbani-Chadegani, A.; Fani, N.; Abdossamadi, S.; Shahmir, N. Toxic effects of lead and nickel nitrate on rat liver chromatin components. J. Biochem. Mol. Toxicol. 2011, 25, 127–134. [Google Scholar] [CrossRef]
- Blasiak, J.; Kowalik, J. A comparison of the in vitro genotoxicity of tri- and hexavalent chromium. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2000, 469, 135–145. [Google Scholar] [CrossRef]
- Zhai, X.W.; Zhang, Y.L.; Qi, Q.; Bai, Y.; Chen, X.L.; Jin, L.J.; Ma, X.G.; Shu, R.Z.; Yang, Z.J.; Liu, F.J. Effects of molybdenum on sperm quality and testis oxidative stress. Syst. Biol. Reprod. Med. 2013, 59, 251–255. [Google Scholar] [CrossRef]
- Qiu, S.; Zhao, F.; Tang, X.; Pei, F.; Dong, H.; Zhu, L.; Guo, K. Type-2 cannabinoid receptor regulates proliferation, apoptosis, differentiation, and OPG/RANKL ratio of MC3T3-E1 cells exposed to Titanium particles. Mol. Cell. Biochem. 2015, 399, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Ponti, J.; Sabbioni, E.; Munaro, B.; Broggi, F.; Marmorato, P.; Franchini, F.; Colognato, R.; Rossi, F. Genotoxicity and morphological transformation induced by cobalt nanoparticles and cobalt chloride: An in vitro study in Balb/3T3 mouse fibroblasts. Mutagenesis 2009, 24, 439–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Environmental Protection Agency. Reference Dose (RfD): Description and Use in Health Risk Assessments. Available online: https://www.epa.gov/iris/reference-dose-rfd-description-and-use-health-risk-assessments (accessed on 7 June 2021).
- Institute of Medicine (US) Food and Nutrition Board. Dietary Reference Intakes; National Academies Press: Washington, DC, USA, 1998; ISBN 978-0-309-06348-7. [Google Scholar]
- Institute of Medicine (US) Food and Nutrition Board. What are Dietary Reference Intakes? In Dietary Reference Intakes: A Risk Assessment Model for Establishing Upper Intake Levels for Nutrients; National Academies Press: Washington, DC, USA, 1998. [Google Scholar]
- European Food Safety Authority. Trusted science for safe food. Available online: https://www.efsa.europa.eu/en (accessed on 9 June 2021).
- Institute of Medicine (US) Panel on Micronutrients. Iron. In Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001; pp. 290–394. [Google Scholar]
- Gaby, A.R. “Safe Upper Levels” for Nutritional Supplements: One Giant Step Backward. J. Orthomol. Med. 2003, 18, 126–130. [Google Scholar]
- Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.; Naska, A.; Neuhäuser-Berthold, M.; et al. Scientific Opinion on Dietary Reference Values for iron. EFSA J. 2015, 13. [Google Scholar] [CrossRef]
- Inštitut za Varovanje Zdravja Republike Slovenije. Referenčne Vrednosti za Vnos Vitaminov in Mineralov—Tabelarična Priporočila za Otroke, Mladostnike, Odrasle in Starejše; Inštitut za varovanje zdravja Republike Slovenije: Ljubljana, Slovenia, 2013. [Google Scholar]
- Iron—Registration Dossier—ECHA. Available online: https://echa.europa.eu/sl/registration-dossier/-/registered-dossier/15429 (accessed on 7 June 2021).
- Institute of Medicine (US) Panel on Micronutrients. Arsenic, Boron, Nickel, Silicon, and Vanadium. In Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001; pp. 502–554. [Google Scholar]
- Nickel—Registration Dossier—ECHA. Available online: https://echa.europa.eu/sl/registration-dossier/-/registered-dossier/15544/1/2 (accessed on 7 June 2021).
- Institute of Medicine (US) Panel on Micronutrients. Chromium. In Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001; pp. 197–224. [Google Scholar]
- Titanium—Registration Dossier—ECHA. Available online: https://echa.europa.eu/sl/registration-dossier/-/registered-dossier/15537 (accessed on 7 June 2021).
- Institute of Medicine (US) Panel on Micronutrients. Molybdenum. In Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001; pp. 420–442. [Google Scholar]
- Agostoni, C.; Berni Canani, R.; Fairweather-Tait, S.; Heinonen, M.H.K.; La Vieille, S.; Marchelli, R.; Martin, A.; Naska, A.; Neuhäuser-Berthold, M.G.; Nowicka, Y.S.; et al. Scientific Opinion on Dietary Reference Values for molybdenum. EFSA J. 2013, 11. [Google Scholar] [CrossRef] [Green Version]
- Molybdenum—Registration Dossier—ECHA. Available online: https://echa.europa.eu/sl/registration-dossier/-/registered-dossier/15524/11/?documentUUID=d7d1afd6-94e2-45d8-90fe-71fc15de0917 (accessed on 7 June 2021).
- Cobalt—Registration Dossier—ECHA. Available online: https://echa.europa.eu/sl/registration-dossier/-/registered-dossier/15506/11 (accessed on 7 June 2021).
- Council Directive from 15th July 1980 Relating the Quality of Water Intended for Human Consumption (80/778/EEC). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L:1980:229:TOC (accessed on 7 June 2021).
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on The Quality of Water Intended for Human Consumption (Recast) (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/dir/2020/2184/oj (accessed on 7 June 2021).
- Smith, P.G.P.G.; Scott, J.G.J.G. Dictionary of Water and Waste Management; Elsevier: Oxford, UK, 2005. [Google Scholar]
- Karnam, K.S.; Reddy, A.N.; Manjith, C. Comparison of Metal Ion Release from Different Bracket Archwire Combinations: An in vitro Study. J. Contemp. Dent. Pract. 2012, 13, 376–381. [Google Scholar] [CrossRef]
- Tahmasbi, S.; Ghorbani, M.; Sheikh, T.; Yaghoubnejad, Y. Galvanic Corrosion and Ion Release from Different Orthodontic Brackets and Wires in Acidic Artificial Saliva. J. Dent. Sch. Shahid Beheshti Univ. Med. Sci. 2019, 32, 37–44. [Google Scholar] [CrossRef]
- Gopikrishnan, S.; Melath, A.; Ajith, V.V.; Mathews, N.B. A comparative study of bio degradation of various orthodontic arch wires: An in vitro study. J. Int. Oral Health 2015, 7, 12–17. [Google Scholar]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Poljšak, B.; Fink, R. The protective role of antioxidants in the defence against ROS/RNS-mediated environmental pollution. Oxidative Med. Cell. Longev. 2014, 2014, 671539. [Google Scholar] [CrossRef] [Green Version]
- Bjelakovic, G.; Nikolova, D.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of gastrointestinal cancers: A systematic review and meta-analysis. Lancet 2004, 364, 1219–1228. [Google Scholar] [CrossRef]
- Miller, E.R.; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Vivekananthan, D.P.; Penn, M.S.; Sapp, S.K.; Hsu, A.; Topol, E.J. Use of antioxidant vitamins for the prevention of cardiovascular disease: Meta-analysis of randomised trials. Lancet 2003, 361, 2017–2023. [Google Scholar] [CrossRef]
- Caraballoso, M.; Sacristan, M.; Serra, C.; Cosp, X.B. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst. Rev. 2003, 2. [Google Scholar] [CrossRef]
- Rhee, S.G. Redox signaling: Hydrogen peroxide as intracellular messenger. Exp. Mol. Med. 1999, 31, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Poljšak, B.; Raspor, P. The antioxidant and pro-oxidant activity of vitamin C and trolox in vitro: A comparative study. J. Appl. Toxicol. 2008, 28, 183–188. [Google Scholar] [CrossRef]
- Poljšak, B.; Gazdag, Z.; Jenko-Brinovec, Š.; Fujs, Š.; Pesti, M.; Bélagyi, J.; Plesničar, S.; Raspor, P. Pro-oxidative vs antioxidative properties of ascorbic acid in chromium(VI)-induced damage: An in vivo and in vitro approach. J. Appl. Toxicol. 2005, 25, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, K.H.; Slater, T.F. An introduction to free radical biochemistry. Br. Med. Bull. 1993, 49, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argüelles, S.; Gómez, A.; Machado, A.; Ayala, A. A preliminary analysis of within-subject variation in human serum oxidative stress parameters as a function of time. Rejuvenation Res. 2007, 10, 621–636. [Google Scholar] [CrossRef]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florêncio, M.H.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.N.I.; Mitsuhashi, S.; Sigetomi, K.; Ubukata, M. Quercetin inhibits advanced glycation end product formation via chelating metal ions, trapping methylglyoxal, and trapping reactive oxygen species. Biosci. Biotechnol. Biochem. 2017, 81, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Chen, C.; Shi, H.; Yang, M.; Liu, Y.; Ji, P.; Chen, H.; Tan, R.X.; Li, E. Curcumin is a biologically active copper chelator with antitumor activity. Phytomedicine 2016, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zieniewska, I.; Maciejczyk, M.; Zalewska, A. The effect of selected dental materials used in conservative dentistry, endodontics, surgery, and orthodontics as well as during the periodontal treatment on the redox balance in the oral cavity. Int. J. Mol. Sci. 2020, 21, 9684. [Google Scholar] [CrossRef] [PubMed]
- Żukowski, P.; Maciejczyk, M.; Waszkiel, D. Sources of free radicals and oxidative stress in the oral cavity. Arch. Oral Biol. 2018, 92, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, G.M.; Gagliano, N.; Zarbin, L.; Tolomeo, G.; Sforza, C. Antioxidant capacity of human saliva and periodontal screening assessment in healthy adults. Arch. Oral Biol. 2017, 78, 34–38. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Primožič, J.; Poljšak, B.; Jamnik, P.; Kovač, V.; Čanadi Jurešić, G.; Spalj, S. Risk Assessment of Oxidative Stress Induced by Metal Ions Released from Fixed Orthodontic Appliances during Treatment and Indications for Supportive Antioxidant Therapy: A Narrative Review. Antioxidants 2021, 10, 1359. https://doi.org/10.3390/antiox10091359
Primožič J, Poljšak B, Jamnik P, Kovač V, Čanadi Jurešić G, Spalj S. Risk Assessment of Oxidative Stress Induced by Metal Ions Released from Fixed Orthodontic Appliances during Treatment and Indications for Supportive Antioxidant Therapy: A Narrative Review. Antioxidants. 2021; 10(9):1359. https://doi.org/10.3390/antiox10091359
Chicago/Turabian StylePrimožič, Jasmina, Borut Poljšak, Polona Jamnik, Vito Kovač, Gordana Čanadi Jurešić, and Stjepan Spalj. 2021. "Risk Assessment of Oxidative Stress Induced by Metal Ions Released from Fixed Orthodontic Appliances during Treatment and Indications for Supportive Antioxidant Therapy: A Narrative Review" Antioxidants 10, no. 9: 1359. https://doi.org/10.3390/antiox10091359
APA StylePrimožič, J., Poljšak, B., Jamnik, P., Kovač, V., Čanadi Jurešić, G., & Spalj, S. (2021). Risk Assessment of Oxidative Stress Induced by Metal Ions Released from Fixed Orthodontic Appliances during Treatment and Indications for Supportive Antioxidant Therapy: A Narrative Review. Antioxidants, 10(9), 1359. https://doi.org/10.3390/antiox10091359