Exploiting Food-Grade Mesoporous Silica to Preserve the Antioxidant Properties of Fresh Olive Mill Wastewaters Phenolic Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Phenolic Extracts and HPLC Analysis
2.3. Preparation and Morphological Characterization of the Inclusion Complexes
2.4. Extraction of Phenolic Compounds
2.5. Total Phenol Content (TPC)
2.6. Ferric Reducing Antioxidant Power (FRAP) Assay
2.7. Radical Scavenging Capacity by DPPH Method
2.8. Cell Culture
2.9. Evaluation of Cell Survival
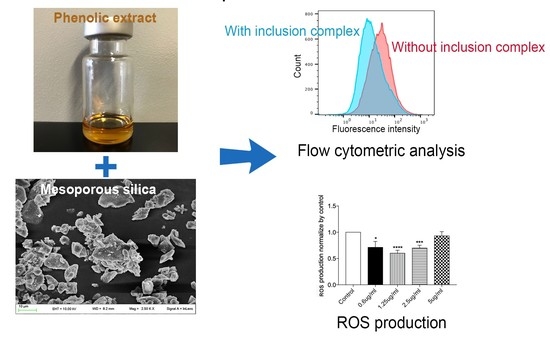
2.10. Evaluation of Antioxidative Activity
2.11. Statistical Analysis
3. Results and Discussion
3.1. HPLC Characterization of Phenolic Extracts
3.2. Phenolic Extract Inclusion Complexes
3.3. Total Phenol Content
3.4. Analyses of Biological Properties of Syl-E and Lip-Syl-E Formulated Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caporaso, N.; Formisano, D.; Genovese, A. Use of Phenolic Compounds from Olive Mill Wastewater as Valuable Ingredients for Functional Foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2829–2841. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.; Morozzi, G. Health and Sensory Properties of Virgin Olive Oil Hydrophilic Phenols: Agronomic and Technological Aspects of Production That Affect Their Occurrence in the Oil. J. Chromatogr. A 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Veneziani, G.; Novelli, E.; Esposto, S.; Taticchi, A.; Servili, M. Chapter 11–Applications of recovered bioactive compounds in food products. In Olive Mill Waste; Galanakis, C.M., Ed.; Academic Press: Chambridge, MA, USA, 2017; pp. 231–253. ISBN 978-0-12-805314-0. [Google Scholar]
- Menchetti, L.; Taticchi, A.; Esposto, S.; Servili, M.; Ranucci, D.; Branciari, R.; Miraglia, D. The Influence of Phenolic Extract from Olive Vegetation Water and Storage Temperature on the Survival of Salmonella Enteritidis Inoculated on Mayonnaise. LWT 2020, 129, 109648. [Google Scholar] [CrossRef]
- Miraglia, D.; Castrica, M.; Menchetti, L.; Esposto, S.; Branciari, R.; Ranucci, D.; Urbani, S.; Sordini, B.; Veneziani, G.; Servili, M. Effect of an Olive Vegetation Water Phenolic Extract on the Physico-Chemical, Microbiological and Sensory Traits of Shrimp (Parapenaeus Longirostris) during the Shelf-Life. Foods 2020, 9, 1647. [Google Scholar] [CrossRef]
- Balzan, S.; Taticchi, A.; Cardazzo, B.; Urbani, S.; Servili, M.; Di Lecce, G.; Zabalza, I.B.; Rodriguez-Estrada, M.T.; Novelli, E.; Fasolato, L. Effect of Phenols Extracted from a By-Product of the Oil Mill on the Shelf-Life of Raw and Cooked Fresh Pork Sausages in the Absence of Chemical Additives. LWT-Food Sci. Tech. 2017, 85, 89–95. [Google Scholar] [CrossRef]
- Pampuri, A.; Casson, A.; Alamprese, C.; Di Mattia, C.D.; Piscopo, A.; Difonzo, G.; Conte, P.; Paciulli, M.; Tugnolo, A.; Beghi, R.; et al. Environmental Impact of Food Preparations Enriched with Phenolic Extracts from Olive Oil Mill Waste. Foods 2021, 10, 980. [Google Scholar] [CrossRef]
- Servili, M.; Rizzello, C.G.; Taticchi, A.; Esposto, S.; Urbani, S.; Mazzacane, F.; Di Maio, I.; Selvaggini, R.; Gobbetti, M.; Di Cagno, R. Functional Milk Beverage Fortified with Phenolic Compounds Extracted from Olive Vegetation Water and Fermented with Functional Lactic Acid Bacteria. Int. J. Food Microbiol. 2011, 147, 45–52. [Google Scholar] [CrossRef]
- Obied, H.K.; Bedgood, D.R.; Prenzler, P.D.; Robards, K. Effect of Processing Conditions, Prestorage Treatment, and Storage Conditions on the Phenol Content and Antioxidant Activity of Olive Mill Waste. J. Agric. Food Chem. 2008, 56, 3925–3932. [Google Scholar] [CrossRef]
- Obied, H.K.; Prenzler, P.D.; Ryan, D.; Servili, M.; Taticchi, A.; Esposto, S.; Robards, K. Biosynthesis and Biotransformations of Phenol-Conjugated Oleosidic Secoiridoids from Olea Europaea L. Nat. Prod. Rep. 2008, 25, 1167–1179. [Google Scholar] [CrossRef]
- Scientific Concepts of Functional Foods in Europe. Consensus Document. Br. J. Nutr. 1999, 81 (Suppl. 1), S1–S27. [Google Scholar]
- Brezoiu, A.-M.; Matei, C.; Deaconu, M.; Stanciuc, A.-M.; Trifan, A.; Gaspar-Pintiliescu, A.; Berger, D. Polyphenols Extract from Grape Pomace. Characterization and Valorisation through Encapsulation into Mesoporous Silica-Type Matrices. Food Chem. Toxicol. 2019, 133, 110787. [Google Scholar] [CrossRef]
- Selvaggini, R.; Esposto, S.; Taticchi, A.; Urbani, S.; Veneziani, G.; Di Maio, I.; Sordini, B.; Servili, M. Optimization of the Temperature and Oxygen Concentration Conditions in the Malaxation during the Oil Mechanical Extraction Process of Four Italian Olive Cultivars. J. Agric. Food Chem. 2014, 62, 3813–3822. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Baldioli, M.; Selvaggini, R.; Macchioni, A.; Montedoro, G. Phenolic Compounds of Olive Fruit: One- and Two-Dimensional Nuclear Magnetic Resonance Characterization of Nüzhenide and Its Distribution in the Constitutive Parts of Fruit. J. Agric. Food Chem. 1999, 47, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Esposto, S.; Veneziani, G.; Urbani, S.; Taticchi, A.; Di Maio, I.; Selvaggini, R.; Sordini, B.; Montedoro, G. Improvement of Bioactive Phenol Content in Virgin Olive Oil with an Olive-Vegetation Water Concentrate Produced by Membrane Treatment. Food Chem. 2011, 124, 1308–1315. [Google Scholar] [CrossRef]
- Esposto, S.; Taticchi, A.; Di Maio, I.; Urbani, S.; Veneziani, G.; Selvaggini, R.; Sordini, B.; Servili, M. Effect of an Olive Phenolic Extract on the Quality of Vegetable Oils during Frying. Food Chem. 2015, 176, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Kettiger, H.; Schoubben, A.; Rosenholm, J.M.; Ambrogi, V.; Hamidi, M. Mesoporous Silica Materials: From Physico-Chemical Properties to Enhanced Dissolution of Poorly Water-Soluble Drugs. J. Control. Release 2017, 262, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Stenger Moura, F.C.; Perioli, L.; Pagano, C.; Vivani, R.; Ambrogi, V.; Bresolin, T.M.; Ricci, M.; Schoubben, A. Chitosan Composite Microparticles: A Promising Gastroadhesive System for Taxifolin. Carbohydr. Polym. 2019, 218, 343–354. [Google Scholar] [CrossRef]
- Ambrogi, V.; Latterini, L.; Marmottini, F.; Pagano, C.; Ricci, M. Mesoporous Silicate MCM-41 as a Particulate Carrier for Octyl Methoxycinnamate: Sunscreen Release and Photostability. J. Pharm. Sci. 2013, 102, 1468–1475. [Google Scholar] [CrossRef]
- Pucciarini, L.; Ianni, F.; Petesse, V.; Pellati, F.; Brighenti, V.; Volpi, C.; Gargaro, M.; Natalini, B.; Clementi, C.; Sardella, R. Onion (Allium Cepa L.) Skin: A Rich Resource of Biomolecules for the Sustainable Production of Colored Biofunctional Textiles. Molecules 2019, 24, 634. [Google Scholar] [CrossRef] [Green Version]
- Lisanti, A.; Formica, V.; Ianni, F.; Albertini, B.; Marinozzi, M.; Sardella, R.; Natalini, B. Antioxidant Activity of Phenolic Extracts from Different Cultivars of Italian Onion (Allium Cepa) and Relative Human Immune Cell Proliferative Induction. Pharm. Biol. 2016, 54, 799–806. [Google Scholar] [CrossRef] [Green Version]
- Rosati, E.; Sabatini, R.; Ayroldi, E.; Tabilio, A.; Bartoli, A.; Bruscoli, S.; Simoncelli, C.; Rossi, R.; Marconi, P. Apoptosis of Human Primary B Lymphocytes Is Inhibited by N-Acetyl-L-Cysteine. J. Leukocyte Biol. 2004, 76, 152–161. [Google Scholar] [CrossRef]
- Hoppstädter, J.; Hachenthal, N.; Valbuena-Perez, J.V.; Lampe, S.; Astanina, K.; Kunze, M.M.; Bruscoli, S.; Riccardi, C.; Schmid, T.; Diesel, B.; et al. Induction of Glucocorticoid-Induced Leucine Zipper (GILZ) Contributes to Anti-Inflammatory Effects of the Natural Product Curcumin in Macrophages. J. Biol. Chem. 2016, 291, 22949–22960. [Google Scholar] [CrossRef] [Green Version]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K.; Stockmann, R. Bioactivity and Analysis of Biophenols Recovered from Olive Mill Waste. J. Agric. Food Chem. 2005, 53, 823–837. [Google Scholar] [CrossRef]
- Hussain, T.; Waters, L.J.; Parkes, G.M.B.; Shahzad, Y. Microwave Processed Solid Dispersions for Enhanced Dissolution of Gemfibrozil Using Non-Ordered Mesoporous Silica. Colloid. Surf. A 2017, 520, 428–435. [Google Scholar] [CrossRef]
- Limnell, T.; Santos, H.A.; Mäkilä, E.; Heikkilä, T.; Salonen, J.; Murzin, D.Y.; Kumar, N.; Laaksonen, T.; Peltonen, L.; Hirvonen, J. Drug Delivery Formulations of Ordered and Nonordered Mesoporous Silica: Comparison of Three Drug Loading Methods. J. Pharm. Sci. 2011, 100, 3294–3306. [Google Scholar] [CrossRef]
- Waters, L.J.; Hanrahan, J.P.; Tobin, J.M.; Finch, C.V.; Parkes, G.M.B.; Ahmad, S.A.; Mohammad, F.; Saleem, M. Enhancing the Dissolution of Phenylbutazone Using Syloid® Based Mesoporous Silicas for Oral Equine Applications. J. Pharm. Anal. 2018, 8, 181–186. [Google Scholar] [CrossRef]
- Ruiz-Rico, M.; Pérez-Esteve, É.; Lerma-García, M.J.; Marcos, M.D.; Martínez-Máñez, R.; Barat, J.M. Protection of Folic Acid through Encapsulation in Mesoporous Silica Particles Included in Fruit Juices. Food. Chem. 2017, 218, 471–478. [Google Scholar] [CrossRef]
- Kreatsouli, K.; Fousteri, Z.; Zampakas, K.; Kerasioti, E.; Veskoukis, A.S.; Mantas, C.; Gkoutsidis, P.; Ladas, D.; Petrotos, K.; Kouretas, D.; et al. A Polyphenolic Extract from Olive Mill Wastewaters Encapsulated in Whey Protein and Maltodextrin Exerts Antioxidant Activity in Endothelial Cells. Antioxidants 2019, 8, 280. [Google Scholar] [CrossRef] [Green Version]
- Schoubben, A.; Vivani, R.; Paolantoni, M.; Perinelli, D.R.; Gioiello, A.; Macchiarulo, A.; Ricci, M. D-Leucine Microparticles as an Excipient to Improve the Aerosolization Performances of Dry Powders for Inhalation. Eur. J. Pharm. Sci. 2019, 130, 54–64. [Google Scholar] [CrossRef]
- 5.4 Residual solvents. In European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2020; pp. 717–724.
- Ambrogi, V.; Marmottini, F.; Pagano, C. Amorphous Carbamazepine Stabilization by the Mesoporous Silicate SBA-15. Micropor. Mesopor. Mater. 2013, 177, 1–7. [Google Scholar] [CrossRef]
- Han, C.; Huang, H.; Dong, Y.; Sui, X.; Jian, B.; Zhu, W. A Comparative Study of the Use of Mesoporous Carbon and Mesoporous Silica as Drug Carriers for Oral Delivery of the Water-Insoluble Drug Carvedilol. Molecules 2019, 24, 1770. [Google Scholar] [CrossRef] [Green Version]
- Breen, S.P.; Etter, N.M.; Ziegler, G.R.; Hayes, J.E. Oral Somatosensatory Acuity Is Related to Particle Size Perception in Chocolate. Sci. Rep. 2019, 9, 7437. [Google Scholar] [CrossRef] [PubMed]
- López-Froilán, R.; Hernández-Ledesma, B.; Cámara, M.; Pérez-Rodríguez, M.L. Evaluation of the Antioxidant Potential of Mixed Fruit-Based Beverages: A New Insight on the Folin-Ciocalteu Method. Food Anal. Methods 2018, 11, 2897–2906. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. A Thorough Study of Reactivity of Various Compound Classes towards the Folin-Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [Green Version]
- DeLany, J.P.; Windhauser, M.M.; Champagne, C.M.; Bray, G.A. Differential Oxidation of Individual Dietary Fatty Acids in Humans. Am. J. Clin. Nutr. 2000, 72, 905–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaich, K.M.; Shahidi, F.; Zhong, Y.; Eskin, N.A.M. Chapter 11–Lipid Oxidation. In Biochemistry of Foods, 3rd ed.; Eskin, N.A.M., Shahidi, F., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 419–478. ISBN 978-0-12-242352-9. [Google Scholar]
- Shahidi, F.; Zhong, Y. Lipid Oxidation and Improving the Oxidative Stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef] [PubMed]




| Compounds | E | M5 | M2 | |||
|---|---|---|---|---|---|---|
| Average | RDS% | Average | RDS% | Average | RDS% | |
| 3,4-DHPEA | 70.40 a | 0.10 | 109.00 b | 0.50 | 105.70 c | 0.30 |
| p-HPEA | 11.40 a | 0.10 | 20.20 b | 0.01 | 23.90 c | 0.03 |
| Vanillic acid | 0.78 | 0.01 | n.d. | n.d. | ||
| Verbascoside | 25.60 a | 1.90 | 33.20 b | 1.50 | 30.90 a,b | 1.30 |
| 3,4-DHPEA-EDA | 479.30 a | 0.90 | 325.40 b | 1.30 | 318.50 c | 0.50 |
| p-HPEA-EDA | 4.90 | 0.80 | n.d. | n.d. | ||
| Sum of phenolic fractions | 592.40 a | 0.70 | 487.90 b | 0.90 | 479.00 b | 0.40 |
| Sample | Entry | Phenol Content (mg eq. GA/mg Matrix y) | Content % | |
|---|---|---|---|---|
| Average | RSD% | |||
| M2 | a | 0.24 b | 0.04 | 100 |
| M5 | b | 0.22 c | 0.06 | 100 |
| E | c | 0.28 a | 0.02 | 100 |
| Syl-M2 | d | 0.08 d,e | 0.09 | 31 |
| Syl-M5 | e | 0.08 f | 0.04 | 34 |
| Syl-E | f | 0.07 | 0.09 | 24 |
| Lip-Syl-M2 | g | 0.04 h | 0.11 | 18 |
| Lip-Syl-M5 | h | 0.04 k | 0.13 | 16 |
| Lip-Syl-E | i | 0.04 | 0.11 | 15 |
| Syl-M2, 22 °C, 75% RH | j | 0.09 d | 10.84 | 35 |
| Syl-M5, 22 °C, 75% RH | k | 0.08 f | 3.97 | 35 |
| Syl-M2, 37 °C, 75% RH | l | 0.06 e | 4.34 | 26 |
| Syl-M5, 37 °C, 75% RH | m | 0.07 g | 8.35 | 29 |
| Lip-Syl-M2, 22 °C, 75% RH | n | 0.05 i | 1.74 | 22 |
| Lip-Syl-M5, 22 °C, 75% RH | o | 0.05 l | 0.64 | 24 |
| Lip-Syl-M2, 37 °C, 75% RH | p | 0.01 j | 11.63 | 5 |
| Lip-Syl-M5, 37 °C, 75% RH | q | 0.02 m | 14.41 | 7 |
| Sample | Entry | FRAP Assay (mg eq. Trolox/mg Matrix y) | Content % | |
|---|---|---|---|---|
| Average | RDS% | |||
| E | a | 0.35 | 0.05 | 100 |
| Syl-E | b | 0.09 | 0.23 | 25 |
| Lip-Syl-E | c | 0.05 | 0.17 | 14 |
| Sample | Entry | DPPH Assay (mg eq. Trolox/mg Matrix y) | Content % | |
|---|---|---|---|---|
| Average | RSD% | |||
| E | a | 0.21 | 0.49 | 100 |
| Syl-E | b | 0.05 | 0.39 | 24 |
| Lip-Syl-E | c | 0.03 | 0.62 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ianni, F.; Gagliardi, A.; Taticchi, A.; Servili, M.; Pinna, N.; Schoubben, A.; Sardella, R.; Bruscoli, S. Exploiting Food-Grade Mesoporous Silica to Preserve the Antioxidant Properties of Fresh Olive Mill Wastewaters Phenolic Extracts. Antioxidants 2021, 10, 1361. https://doi.org/10.3390/antiox10091361
Ianni F, Gagliardi A, Taticchi A, Servili M, Pinna N, Schoubben A, Sardella R, Bruscoli S. Exploiting Food-Grade Mesoporous Silica to Preserve the Antioxidant Properties of Fresh Olive Mill Wastewaters Phenolic Extracts. Antioxidants. 2021; 10(9):1361. https://doi.org/10.3390/antiox10091361
Chicago/Turabian StyleIanni, Federica, Andrea Gagliardi, Agnese Taticchi, Maurizio Servili, Nicola Pinna, Aurélie Schoubben, Roccaldo Sardella, and Stefano Bruscoli. 2021. "Exploiting Food-Grade Mesoporous Silica to Preserve the Antioxidant Properties of Fresh Olive Mill Wastewaters Phenolic Extracts" Antioxidants 10, no. 9: 1361. https://doi.org/10.3390/antiox10091361
APA StyleIanni, F., Gagliardi, A., Taticchi, A., Servili, M., Pinna, N., Schoubben, A., Sardella, R., & Bruscoli, S. (2021). Exploiting Food-Grade Mesoporous Silica to Preserve the Antioxidant Properties of Fresh Olive Mill Wastewaters Phenolic Extracts. Antioxidants, 10(9), 1361. https://doi.org/10.3390/antiox10091361