Effects of Hyperbaric Oxygen Preconditioning on Doxorubicin Cardiorespiratory Toxicity
Abstract
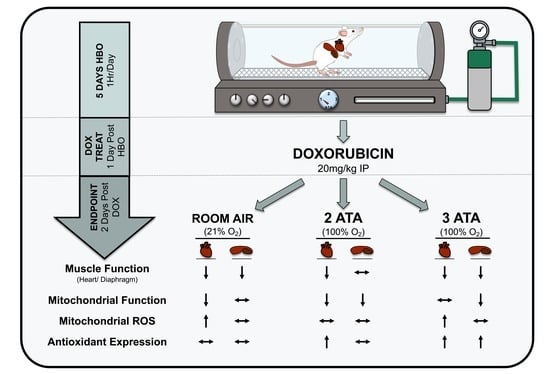
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Hyperbaric Oxygen Treatment
2.3. DOX Treatment
2.4. Echocardiography
2.5. Plasma Collection and Analysis
2.6. Diaphragm Force Production and Fatigue
2.7. Diaphragm Cross-Sectional Area Analysis
2.8. Permeabilized Muscle Fibers, Mitochondrial Respiration and ROS Emission
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Plasma Cytokine/Chemokine Levels Pre- and Post-Acute HBO Exposure
3.2. Body Weight and Heart Weight Are Decreased with DOX Treatment
3.3. Cardiac Function Is Reduced with DOX and Not Improved by HBO Preconditioning
3.4. Diaphragm Dysfunction Is Observed Following DOX Treatment
3.5. DOX Promotes Mitochondrial Dysfunction and Reactive Oxygen Species Production
3.6. HBO Preconditioning Increases Muscle Antioxidant Capacity
3.7. Alterations in Circulating Chemokines/Cytokines
4. Discussion
4.1. Inflammation, Oxidative Stress and Mitochondrial Function
4.2. HBO Preconditioning and Cardiorespiratory Muscle Function
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baude, J.; Cooper, J.S. Hyperbaric contraindicated chemotherapeutic agents. In StatPearls; StatsPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Moon, R.E. Hyperbaric oxygen treatment for decompression sickness. Undersea Hyperb. Med. 2014, 41, 151–157. [Google Scholar] [PubMed]
- Bhutani, S.; Vishwanath, G. Hyperbaric oxygen and wound healing. Indian J. Plast. Surg. 2012, 45, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R. Analytic Reviews: Hyperbaric Oxygen Therapy. J. Intensiv. Care Med. 1989, 4, 58–74. [Google Scholar] [CrossRef]
- Smuder, A.J.; Turner, S.M.; Schuster, C.M.; Morton, A.B.; Hinkley, J.M.; Fuller, D.D. Hyperbaric Oxygen Treatment Following Mid-Cervical Spinal Cord Injury Preserves Diaphragm Muscle Function. Int. J. Mol. Sci. 2020, 21, 7219. [Google Scholar] [CrossRef]
- Gregorevic, P.; Lynch, G.S.; Williams, D.A. Hyperbaric oxygen modulates antioxidant enzyme activity in rat skeletal muscles. Eur. J. Appl. Physiol. 2001, 86, 24–27. [Google Scholar] [CrossRef]
- Gregorevic, P.; Williams, D.A.; Lynch, G.S. Hyperbaric oxygen increases the contractile function of regenerating rat slow muscles. Med. Sci. Sports Exerc. 2002, 34, 630–636. [Google Scholar] [CrossRef]
- Gregorevic, P.; Lynch, G.S.; Williams, D.A. Hyperbaric oxygen improves contractile function of regenerating rat skeletal muscle after myotoxic injury. J. Appl. Physiol. 2000, 89, 1477–1482. [Google Scholar] [CrossRef]
- Oyaizu, T.; Enomoto, M.; Yamamoto, N.; Tsuji, K.; Horie, M.; Muneta, T.; Sekiya, I.; Okawa, A.; Yagishita, K. Hyperbaric oxygen reduces inflammation, oxygenates injured muscle, and regenerates skeletal muscle via macrophage and satellite cell activation. Sci. Rep. 2018, 8, 1288. [Google Scholar] [CrossRef] [Green Version]
- Hadanny, A.; Hachmo, Y.; Rozali, D.; Catalogna, M.; Yaakobi, E.; Sova, M.; Gattegno, H.; Abu Hamed, R.; Lang, E.; Polak, N.; et al. Effects of Hyperbaric Oxygen Therapy on Mitochondrial Respiration and Physical Performance in Middle-Aged Athletes: A Blinded, Randomized Controlled Trial. Sports Med. Open 2022, 8, 22. [Google Scholar] [CrossRef]
- Smuder, A.J. Exercise stimulates beneficial adaptations to diminish doxorubicin-induced cellular toxicity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R662–R672. [Google Scholar] [CrossRef]
- Montalvo, R.N.; Doerr, V.; Nguyen, B.L.; Kelley, R.C.; Smuder, A.J. Consideration of Sex as a Biological Variable in the Development of Doxorubicin Myotoxicity and the Efficacy of Exercise as a Therapeutic Intervention. Antioxidants 2021, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.B.; Sardao, V.A.; Oliveira, P.J. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ. Res. 2020, 126, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.B. Doxorubicin-induced cardiac mitochondrionopathy. Pharmacol. Toxicol. 2003, 93, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Leitman, M.; Efrati, S.; Fuchs, S.; Hadanny, A.; Vered, Z. The effect of hyperbaric oxygenation therapy on myocardial function. Int. J. Cardiovasc. Imaging 2020, 36, 833–840. [Google Scholar] [CrossRef]
- Wunderlich, T.; Frey, N.; Kahler, W.; Lutz, M.; Radermacher, P.; Klapa, S.; Koch, I.; Tillmans, F.; Witte, J.; Koch, A. Influence of hyperoxia on diastolic myocardial and arterial endothelial function. Undersea Hyperb. Med. 2017, 44, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Yogaratnam, J.Z.; Laden, G.; Guvendik, L.; Cowen, M.; Cale, A.; Griffin, S. Hyperbaric oxygen preconditioning improves myocardial function, reduces length of intensive care stay, and limits complications post coronary artery bypass graft surgery. Cardiovasc. Revascularization Med. 2010, 11, 8–19. [Google Scholar] [CrossRef]
- Nie, H.; Xiong, L.; Lao, N.; Chen, S.; Xu, N.; Zhu, Z. Hyperbaric oxygen preconditioning induces tolerance against spinal cord ischemia by upregulation of antioxidant enzymes in rabbits. J. Cereb. Blood Flow Metab. 2006, 26, 666–674. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.H.; Choi, H.; Chun, Y.S.; Kim, G.T.; Park, J.W.; Kim, M.S. Hyperbaric oxygenation pretreatment induces catalase and reduces infarct size in ischemic rat myocardium. Pflug. Arch. 2001, 442, 519–525. [Google Scholar] [CrossRef]
- Yu, S.Y.; Chiu, J.H.; Yang, S.D.; Yu, H.Y.; Hsieh, C.C.; Chen, P.J.; Lui, W.Y.; Wu, C.W. Preconditioned hyperbaric oxygenation protects the liver against ischemia-reperfusion injury in rats. J. Surg. Res. 2005, 128, 28–36. [Google Scholar] [CrossRef]
- Cabigas, B.P.; Su, J.; Hutchins, W.; Shi, Y.; Schaefer, R.B.; Recinos, R.F.; Nilakantan, V.; Kindwall, E.; Niezgoda, J.A.; Baker, J.E. Hyperoxic and hyperbaric-induced cardioprotection: Role of nitric oxide synthase 3. Cardiovasc. Res. 2006, 72, 143–151. [Google Scholar] [CrossRef]
- Tezcan, O.; Karahan, O.; Alan, M.; Ekinci, C.; Yavuz, C.; Demirtas, S.; Ekinci, A.; Caliskan, A. Hyperbaric Oxygen Preconditioning Provides Preliminary Protection Against Doxorubicin Cardiotoxicity. Acta Cardiol. Sin. 2017, 33, 150–155. [Google Scholar] [CrossRef]
- Karagoz, B.; Suleymanoglu, S.; Uzun, G.; Bilgi, O.; Aydinoz, S.; Haholu, A.; Turken, O.; Onem, Y.; Kandemir, E.G. Hyperbaric oxygen therapy does not potentiate doxorubicin-induced cardiotoxicity in rats. Basic Clin. Pharmacol. Toxicol. 2008, 102, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Upton, P.G.; Yamaguchi, K.T.; Myers, S.; Kidwell, T.P.; Anderson, R.J. Effects of antioxidants and hyperbaric oxygen in ameliorating experimental doxorubicin skin toxicity in the rat. Cancer Treat. Rep. 1986, 70, 503–507. [Google Scholar]
- Firat, O.; Kirdok, O.; Makay, O.; Caliskan, C.; Yilmaz, F.; Ilgezdi, S.; Karabulut, B.; Coker, A.; Zeytunlu, M. Can hyperbaric oxygenation decrease doxorubicin hepatotoxicity and improve regeneration in the injured liver? J. Hepato-Biliary-Pancreat. Surg. 2009, 16, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Aktas, S.; Toklu, A.S.; Olgac, V. Hyperbaric oxygen therapy in adriamycin extravasation: An experimental animal study. Ann. Plast. Surg. 2000, 45, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Monstrey, S.J.; Mullick, P.; Narayanan, K.; Ramasastry, S.S. Hyperbaric oxygen therapy and free radical production: An experimental study in doxorubicin (Adriamycin) extravasation injuries. Ann. Plast. Surg. 1997, 38, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Thigpen, J.T.; Brady, M.F.; Homesley, H.D.; Malfetano, J.; DuBeshter, B.; Burger, R.A.; Liao, S. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: A gynecologic oncology group study. J. Clin. Oncol. 2004, 22, 3902–3908. [Google Scholar] [CrossRef]
- Cai, F.; Luis, M.A.F.; Lin, X.; Wang, M.; Cai, L.; Cen, C.; Biskup, E. Anthracycline-induced cardiotoxicity in the chemotherapy treatment of breast cancer: Preventive strategies and treatment. Mol. Clin. Oncol. 2019, 11, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Tibbles, P.M.; Edelsberg, J.S. Hyperbaric-oxygen therapy. N. Engl. J. Med. 1996, 334, 1642–1648. [Google Scholar] [CrossRef]
- Montalvo, R.N.; Doerr, V.; Kwon, O.S.; Talbert, E.E.; Yoo, J.K.; Hwang, M.H.; Nguyen, B.L.; Christou, D.D.; Kavazis, A.N.; Smuder, A.J. Protection against Doxorubicin-Induced Cardiac Dysfunction Is Not Maintained Following Prolonged Autophagy Inhibition. Int. J. Mol. Sci. 2020, 21, 8105. [Google Scholar] [CrossRef]
- Powers, S.K.; Shanely, R.A.; Coombes, J.S.; Koesterer, T.J.; McKenzie, M.; Van Gammeren, D.; Cicale, M.; Dodd, S.L. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J. Appl. Physiol. 2002, 92, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B. Free radicals and muscle fatigue: Of ROS, canaries, and the IOC. Free Radic. Biol. Med. 2008, 44, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Smuder, A.J.; Sollanek, K.J.; Nelson, W.B.; Min, K.; Talbert, E.E.; Kavazis, A.N.; Hudson, M.B.; Sandri, M.; Szeto, H.H.; Powers, S.K. Crosstalk between autophagy and oxidative stress regulates proteolysis in the diaphragm during mechanical ventilation. Free Radic. Biol. Med. 2018, 115, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Kwon, O.S.; Smuder, A.J.; Wiggs, M.P.; Sollanek, K.J.; Christou, D.D.; Yoo, J.K.; Hwang, M.H.; Szeto, H.H.; Kavazis, A.N.; et al. Increased mitochondrial emission of reactive oxygen species and calpain activation are required for doxorubicin-induced cardiac and skeletal muscle myopathy. J. Physiol. 2015, 593, 2017–2036. [Google Scholar] [CrossRef] [Green Version]
- Smuder, A.J.; Hudson, M.B.; Nelson, W.B.; Kavazis, A.N.; Powers, S.K. Nuclear factor-kappaB signaling contributes to mechanical ventilation-induced diaphragm weakness*. Crit. Care Med. 2012, 40, 927–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilliam, L.A.; Moylan, J.S.; Callahan, L.A.; Sumandea, M.P.; Reid, M.B. Doxorubicin causes diaphragm weakness in murine models of cancer chemotherapy. Muscle Nerve 2011, 43, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Efrati, S.; Gall, N.; Bergan, J.; Fishlev, G.; Bass, A.; Berman, S.; Hamad-Abu, R.; Feigenzon, M.; Weissgarten, J. Hyperbaric oxygen, oxidative stress, NO bioavailability and ulcer oxygenation in diabetic patients. Undersea Hyperb. Med. 2009, 36, 1–12. [Google Scholar]
- Tsuneyama, K.; Chen, Y.C.; Fujimoto, M.; Sasaki, Y.; Suzuki, W.; Shimada, T.; Iizuka, S.; Nagata, M.; Aburada, M.; Chen, S.Y. Advantages and disadvantages of hyperbaric oxygen treatment in mice with obesity hyperlipidemia and steatohepatitis. Sci. World J. 2011, 11, 2124–2135. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.S.; Tanaka, L.Y.; Antonio, E.L.; Brandizzi, L.I.; Serra, A.J.; Dos Santos, L.; Krieger, J.E.; Laurindo, F.R.M.; Tucci, P.J.F. Hyperbaric oxygenation improves redox control and reduces mortality in the acute phase of myocardial infarction in a rat model. Mol. Med. Rep. 2020, 21, 1431–1438. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Zhu, Y.; Huang, W.; Li, J.; Zhang, B.; Li, Z.; Yang, X. Hyperbaric Oxygen Potentiates Doxil Antitumor Efficacy by Promoting Tumor Penetration and Sensitizing Cancer Cells. Adv. Sci 2018, 5, 1700859. [Google Scholar] [CrossRef]
- Heys, S.D.; Smith, I.C.; Ross, J.A.; Gilbert, F.J.; Brooks, J.; Semple, S.; Miller, I.D.; Hutcheon, A.; Sarkar, T.; Eremin, O. A pilot study with long term follow up of hyperbaric oxygen pretreatment in patients with locally advanced breast cancer undergoing neo-adjuvant chemotherapy. Undersea Hyperb. Med. 2006, 33, 33–43. [Google Scholar] [PubMed]
- Benko, R.; Miklos, Z.; Agoston, V.A.; Ihonvien, K.; Repas, C.; Csepanyi-Komi, R.; Kerek, M.; Beres, N.J.; Horvath, E.M. Hyperbaric Oxygen Therapy Dampens Inflammatory Cytokine Production and Does Not Worsen the Cardiac Function and Oxidative State of Diabetic Rats. Antioxidants 2019, 8, 607. [Google Scholar] [CrossRef] [PubMed]
- Schottlender, N.; Gottfried, I.; Ashery, U. Hyperbaric Oxygen Treatment: Effects on Mitochondrial Function and Oxidative Stress. Biomolecules 2021, 11, 1827. [Google Scholar] [CrossRef] [PubMed]
- Hedetoft, M.; Garred, P.; Madsen, M.B.; Hyldegaard, O. Hyperbaric oxygen treatment is associated with a decrease in cytokine levels in patients with necrotizing soft-tissue infection. Physiol. Rep. 2021, 9, e14757. [Google Scholar] [CrossRef]
- Weisz, G.; Lavy, A.; Adir, Y.; Melamed, Y.; Rubin, D.; Eidelman, S.; Pollack, S. Modification of in vivo and in vitro TNF-alpha, IL-1, and IL-6 secretion by circulating monocytes during hyperbaric oxygen treatment in patients with perianal Crohn’s disease. J. Clin. Immunol. 1997, 17, 154–159. [Google Scholar] [CrossRef]
- Dhamodharan, U.; Karan, A.; Sireesh, D.; Vaishnavi, A.; Somasundar, A.; Rajesh, K.; Ramkumar, K.M. Tissue-specific role of Nrf2 in the treatment of diabetic foot ulcers during hyperbaric oxygen therapy. Free Radic. Biol. Med. 2019, 138, 53–62. [Google Scholar] [CrossRef]
- Yin, X.; Wang, X.; Fan, Z.; Peng, C.; Ren, Z.; Huang, L.; Liu, Z.; Zhao, K. Hyperbaric Oxygen Preconditioning Attenuates Myocardium Ischemia-Reperfusion Injury Through Upregulation of Heme Oxygenase 1 Expression: PI3K/Akt/Nrf2 Pathway Involved. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 428–438. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Chen, W.; Li, Y.; Dong, Y.; Teng, X.; Nong, Z.; Pan, X.; Lv, L.; Gao, Y.; Wu, G. Hyperbaric oxygen protects against myocardial reperfusion injury via the inhibition of inflammation and the modulation of autophagy. Oncotarget 2017, 8, 111522–111534. [Google Scholar] [CrossRef] [Green Version]
- Dave, K.R.; Prado, R.; Busto, R.; Raval, A.P.; Bradley, W.G.; Torbati, D.; Perez-Pinzon, M.A. Hyperbaric oxygen therapy protects against mitochondrial dysfunction and delays onset of motor neuron disease in Wobbler mice. Neuroscience 2003, 120, 113–120. [Google Scholar] [CrossRef]
- Zhou, Z.; Daugherty, W.P.; Sun, D.; Levasseur, J.E.; Altememi, N.; Hamm, R.J.; Rockswold, G.L.; Bullock, M.R. Protection of mitochondrial function and improvement in cognitive recovery in rats treated with hyperbaric oxygen following lateral fluid-percussion injury. J. Neurosurg. 2007, 106, 687–694. [Google Scholar] [CrossRef] [Green Version]
- Palzur, E.; Zaaroor, M.; Vlodavsky, E.; Milman, F.; Soustiel, J.F. Neuroprotective effect of hyperbaric oxygen therapy in brain injury is mediated by preservation of mitochondrial membrane properties. Brain Res. 2008, 1221, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Tezgin, D.; Giardina, C.; Perdrizet, G.A.; Hightower, L.E. The effect of hyperbaric oxygen on mitochondrial and glycolytic energy metabolism: The caloristasis concept. Cell Stress Chaperones 2020, 25, 667–677. [Google Scholar] [CrossRef] [PubMed]







| CON | DOX | 2ATA-DOX | 3ATA-DOX | |
|---|---|---|---|---|
| Heart Rate (bpm) | 330.7 ± 13.8 | 347.2 ± 9.4 | 323.1 ± 7.7 | 344.3 ± 9.8 |
| SWTd (mm) | 1.79 ± 0.26 | 1.74 ± 0.11 | 1.61 ± 0.09 | 1.75 ± 0.10 |
| SWTs (mm) | 2.59 ± 0.27 | 2.57 ± 0.17 | 2.09 ± 0.02 | 2.40 ± 0.07 |
| PWTd (mm) | 1.72 ± 0.04 | 1.61 ± 0.05 | 1.60 ± 0.08 | 1.46 ± 0.07 ^ |
| PWTs (mm) | 2.76 ± 0.07 | 2.40 ± 0.08 | 2.32 ± 0.16 ^ | 2.17 ± 0.11 ^ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doerr, V.; Montalvo, R.N.; Nguyen, B.L.; Boeno, F.P.; Sunshine, M.D.; Bindi, V.E.; Fuller, D.D.; Smuder, A.J. Effects of Hyperbaric Oxygen Preconditioning on Doxorubicin Cardiorespiratory Toxicity. Antioxidants 2022, 11, 2073. https://doi.org/10.3390/antiox11102073
Doerr V, Montalvo RN, Nguyen BL, Boeno FP, Sunshine MD, Bindi VE, Fuller DD, Smuder AJ. Effects of Hyperbaric Oxygen Preconditioning on Doxorubicin Cardiorespiratory Toxicity. Antioxidants. 2022; 11(10):2073. https://doi.org/10.3390/antiox11102073
Chicago/Turabian StyleDoerr, Vivian, Ryan N. Montalvo, Branden L. Nguyen, Franccesco P. Boeno, Michael D. Sunshine, Victoria E. Bindi, David D. Fuller, and Ashley J. Smuder. 2022. "Effects of Hyperbaric Oxygen Preconditioning on Doxorubicin Cardiorespiratory Toxicity" Antioxidants 11, no. 10: 2073. https://doi.org/10.3390/antiox11102073
APA StyleDoerr, V., Montalvo, R. N., Nguyen, B. L., Boeno, F. P., Sunshine, M. D., Bindi, V. E., Fuller, D. D., & Smuder, A. J. (2022). Effects of Hyperbaric Oxygen Preconditioning on Doxorubicin Cardiorespiratory Toxicity. Antioxidants, 11(10), 2073. https://doi.org/10.3390/antiox11102073