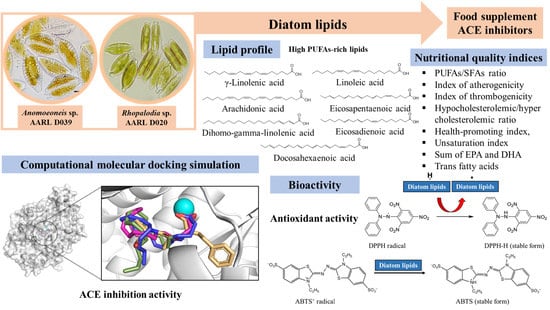
Lipid Profile, Antioxidant and Antihypertensive Activity, and Computational Molecular Docking of Diatom Fatty Acids as ACE Inhibitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diatom Samples
2.2. Lipid Extraction
2.3. Analysis of Fatty Acid Composition
2.4. Evaluation of Nutritional Quality Indices
− 3 PUFA) + (n − 3/n − 6)]
[4 × (%tetraenoics)] + [5 × (%pentaenoics)] + [6 × (%hexaenoics)]
2.5. Bioactivities of Lipid Extract
Determination of Antioxidant Properties
- DPPH radical scavenging activity
- ABTS radical scavenging activity
- Angiotensin-converting enzyme (ACE) inhibitory activity
2.6. Molecular Docking Analysis of ACE
2.6.1. Preparation of Target Protein and Ligands
2.6.2. Molecular Docking Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Diatom Lipids
3.2. Nutritional Quality Indices
3.3. Antioxidant Properties
3.4. Antihypertensive Activity
3.5. Computational Molecular Docking Simulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maneechote, W.; Cheirsilp, B.; Srinuanpan, S.; Pathom-aree, W. Optimizing physicochemical factors for two-stage cultivation of newly isolated oleaginous microalgae from local lake as promising sources of pigments, PUFAs and biodiesel feedstocks. Bioresour. Technol. Rep. 2021, 15, 100738. [Google Scholar] [CrossRef]
- Lomakool, S.; Ruangrit, K.; Jeerapan, I.; Tragoolpua, Y.; Pumas, C.; Srinuanpan, S.; Pekkoh, J.; Duangjan, K. Biological activities and phytochemicals profiling of different cyanobacterial and microalgal biomass. Biomass Convers. Biorefinery 2021, 1–17. [Google Scholar] [CrossRef]
- Govindan, N.; Maniam, G.P.; Sulaiman, A.Z.; Ajit, A.; Chatsungnoen, T.; Chisti, Y. Production of renewable lipids by the diatom Amphora copulate. Fermentation 2021, 7, 37. [Google Scholar] [CrossRef]
- Di Dato, V.; Di Costanzo, F.; Barbarinaldi, R.; Perna, A.; Ianora, A.; Romano, G. Unveiling the presence of biosynthetic pathways for bioactive compounds in the Thalassiosira rotula transcriptome. Sci. Rep. 2019, 9, 9893. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.K.; Seibert, M. Prospects for commercial production of diatoms. Biotechnol. Biofuels 2017, 10, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conde, T.A.; Neves, B.F.; Couto, D.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, M.R. Microalgae as sustainable bio-factories of healthy lipids: Evaluating fatty acid content and antioxidant activity. Mar. Drugs 2021, 19, 357. [Google Scholar] [CrossRef]
- Das, U.N. Long-chain polyunsaturated fatty acids interact with nitric oxide, superoxide anion, and transforming growth factor-β to prevent human essential hypertension. Eur. J. Clin. Nutr. 2004, 58, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Sergeant, S.; Rahbar, E.; Chilton, F.H. Gamma-linolenic acid, dihommo-gamma linolenic, eicosanoids and inflammatory processes. Eur. J. Pharmacol. 2016, 785, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Liu, H. Nutritional indices for assessing fatty acids: A mini-review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Araujo, P.; Truzzi, C.; Belghit, I.; Antonucci, M. The impact of seawater warming on fatty acid composition and nutritional quality indices of Trematomus bernacchii from the Antarctic region. Food Chem. 2021, 365, 130500. [Google Scholar] [CrossRef] [PubMed]
- Kheeree, N.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Choowongkomon, K.; Karnchanatat, A. ACE inhibitory peptides derived from de-fatted lemon basil seeds: Optimization, purification, identification, structure–activity relationship and molecular docking analysis. Food Funct. 2020, 11, 8161–8178. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Mirdamadi, S.; Safavi, M. Structural analysis of ACE-inhibitory peptide (VL-9) derived from Kluyveromyces marxianus protein hydrolysate. J. Mol. Struct. 2020, 1213, 128199. [Google Scholar] [CrossRef]
- Vilakazi, H.; Olasehinde, T.A.; Olaniran, A.O. Chemical characterization, antiproliferative and antioxidant activities of polyunsaturated fatty acid-rich extracts from Chlorella sp. S14. Molecules 2021, 26, 4109. [Google Scholar] [CrossRef]
- Verspreet, J.; Soetemans, L.; Gargan, C.; Hayes, M.; Bastiaens, L. Nutritional Profiling and preliminary bioactivity screening of five micro-algae strains cultivated in Northwest Europe. Foods 2021, 10, 1516. [Google Scholar] [CrossRef] [PubMed]
- Ruangrit, K.; Chaipoot, S.; Phongphisutthinant, R.; Duangjan, K.; Phinyo, K.; Jeerapan, I.; Pekkoh, J.; Srinuanpan, S. A successful biorefinery approach of macroalgal biomass as a promising sustainable source to produce bioactive nutraceutical and biodiesel. Biomass Convers. Biorefinery 2021, 1–11. [Google Scholar] [CrossRef]
- Pekkoh, J.; Ruangrit, K.; Pumas, C.; Duangjan, K.; Chaipoot, S.; Phongphisutthinant, R.; Jeerapan, I.; Sawangrat, K.; Pathom-aree, W.; Srinuanpan, S. Transforming microalgal Chlorella biomass into cosmetically and nutraceutically protein hydrolysates using high-efficiency enzymatic hydrolysis approach. Biomass Convers. Biorefinery 2021, 1–17. [Google Scholar] [CrossRef]
- Caballero, J. Considerations for docking of selective angiotensin-converting enzyme inhibitors. Molecules 2020, 25, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, K.E.; Shen, X.Z.; Gonzalez-Villalobos, R.A.; Billet, S.; Okwan-Duodu, D.; Ong, F.S.; Fuchs, S. Different in vivo functions of the two catalytic domains of angiotensin-converting enzyme (ACE). Curr. Opin. Pharmacol. 2011, 11, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Cozier, G.E.; Arendse, L.B.; Schwager, S.L.; Sturrock, E.D.; Acharya, K.R. Molecular basis for multiple omapatrilat binding sites within the ACE C-domain: Implications for drug design. J. Med. Chem. 2018, 61, 10141–10154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onufriev, A.V.; Alexov, E. Protonation and pK changes in protein–ligand binding. Q. Rev. Biophys. 2013, 46, 181–209. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R. Gaussian 09, Revision B. 01; Gaussian: Wallingford, CT, USA, 2009. [Google Scholar]
- Fields, F.J.; Kociolek, J.P. An evolutionary perspective on selecting high-lipid-content diatoms (Bacillariophyta). J. Appl. Phycol. 2015, 27, 2209–2220. [Google Scholar] [CrossRef]
- Banskota, A.H.; Sperker, S.; Stefanova, R.; McGinn, P.J.; O’Leary, S.J. Antioxidant properties and lipid composition of selected microalgae. J. Appl. Phycol. 2019, 31, 309–318. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of animal and marine origin: Structure, function, and anti-inflammatory properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef] [Green Version]
- Yi, Z.; Xu, M.; Di, X.; Brynjolfsson, S.; Fu, W. Exploring valuable lipids in diatoms. Front. Mar. Sci. 2017, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Das, U.N. Essential fatty acids and their metabolites could function as endogenous HMG-CoA reductase and ACE enzyme inhibitors, anti-arrhythmic, anti-hypertensive, anti-atherosclerotic, anti-inflammatory, cytoprotective, and cardioprotective molecules. Lipids Health Dis. 2008, 7, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blasio, M.; Balzano, S. Fatty acids derivatives from eukaryotic microalgae, pathways and potential applications. Front. Microbiol. 2021, 12, 718933. [Google Scholar] [CrossRef] [PubMed]
- Jusoh, M.; Loh, S.H.; Chuah, T.S.; Aziz, A.; San Cha, T. Indole-3-acetic acid (IAA) induced changes in oil content, fatty acid profiles and expression of four fatty acid biosynthetic genes in Chlorella vulgaris at early stationary growth phase. Phytochemistry 2015, 111, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, M.; Kano, M.A.; Abdullahi, N.; Kankara, I.A.; Ibrahim, S.I.; Muhammad, Y.Y. Extraction, characterization and fatty acids profiles of Nymphaea Lotus and Nymphaea Pubescens seed oils. Biosci. Biotechnol. Res. Asia 2017, 14, 1299–1307. [Google Scholar] [CrossRef]
- Yurchenko, S.; Sats, A.; Tatar, V.; Kaart, T.; Mootse, H.; Jõudu, I. Fatty acid profile of milk from Saanen and Swedish Landrace goats. Food Chem. 2018, 254, 326–332. [Google Scholar] [CrossRef]
- Matos, Â.P.; Feller, R.; Moecke, E.H.S.; de Oliveira, J.V.; Junior, A.F.; Derner, R.B.; Sant’Anna, E.S. Chemical characterization of six microalgae with potential utility for food application. J. Am. Oil Chem. Soc. 2016, 93, 963–972. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; González-Barriga, V.; Romero, J.; Rojas, R.; López-Arana, S. Quantification and distribution of omega-3 fatty acids in South Pacific fish and shellfish species. Foods 2020, 9, 233. [Google Scholar] [CrossRef] [Green Version]
- Buono, S.; Langellotti, A.L.; Martello, A.; Rinna, F.; Fogliano, V. Functional ingredients from microalgae. Food Funct. 2014, 5, 1669–1685. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Drivers of Emerging Risks and Their Interactions in the Domain of Biological Risks to Animal, Plant and Public Health: A Pilot Study; EFSA Supporting Publications: Parma, Italy, 2014; 44p. [Google Scholar]
- Raff, M.; Tholstrup, T.; Sejrsen, K.; Straarup, E.M.; Wiinberg, N. Diets rich in conjugated linoleic acid and vaccenic acid have no effect on blood pressure and isobaric arterial elasticity in healthy young men. J. Nutr. 2006, 136, 992–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Costa, E.; Melo, T.; Reis, M.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Polar lipids composition, antioxidant and anti-inflammatory activities of the atlantic red seaweed Grateloupia turuturu. Mar. Drugs 2021, 19, 414. [Google Scholar] [CrossRef] [PubMed]
- Shetty, V.; Mokashi, K.; Sibi, G. Variations among antioxidant profiles in lipid and phenolic extracts of microalgae from different growth medium. J. Fish. Aquat. Sci. 2015, 10, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Peraman, M.; Nachimuthu, S. Identification and quantification of fucoxanthin in selected carotenoid-producing marine microalgae and evaluation for their chemotherapeutic potential. Pharmacogn. Mag. 2019, 15, 243–249. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, Y.A.; Shin, S.B.; Lee, T.S.; Yoon, H.D. Angiotensin-I converting enzyme fatty acid inhibitory fractions from the Korean melania snail Semisulcospira coreana. Food Sci. Biotechnol. 2015, 24, 681–688. [Google Scholar] [CrossRef]



| FA | 2D Structure | Concentration (%) | |
|---|---|---|---|
| Anomoeoneis Sp. AARL D039 | Rhopalodia Sp. AARL D020 | ||
| Lipid Content (%w/w) | 9.92 ± 1.08 | 12.72 ± 0.90 | |
| Saturated fatty acid (SFA) | |||
| Caproic acid (C6:0) |  | N.D. | 0.07 ± 0.02 |
| Capric acid (C10:0) |  | N.D. | 0.07 ± 0.02 |
| Lauric acid (C12:0) |  | N.D. | 0.12 ± 0.01 |
| Myristic acid (C14:0) |  | 1.72 ± 0.01 | 5.95 ± 0.04 |
| Pentadecanoic acid (C15:0) |  | 0.55 ± 0.04 | 0.43 ± 0.02 |
| Palmitic acid (C16:0) |  | 33.02 ± 0.01 | 31.22 ± 0.01 |
| Margaric acid (C17:0) |  | 0.38 ± 0.01 | 0.25 ± 0.04 |
| Steric acid (C18:0) |  | 5.63 ± 0.02 | 3.30 ± 0.00 |
| Behenic acid (C22:0) |  | 0.23 ± 0.02 | N.D. |
| Lignoceric acid (C24:0) |  | 0.22 ± 0.01 | N.D. |
| Monounsaturated fatty acids (MUFA) | |||
| Myristoleic acid (C14:1) |  | 0.34 ± 0.03 | 0.12 ± 0.01 |
| Palmitoleic acid (C16:1) |  | 34.61 ± 0.01 | 24.67 ± 0.02 |
| Ginkgolic acid (C17:1) |  | 0.80 ± 0.00 | 0.20 ± 0.00 |
| Elaidic acid (C18:1n9t) |  | 2.26 ± 0.03 | 3.14 ± 0.03 |
| Oleic acid (C18:1n9c) |  | 4.30 ± 0.01 | 7.40 ± 0.00 |
| Erucic acid (C22:1n9) |  | 0.23 ± 0.02 | N.D. |
| Nervonic acid (C24:1n9) |  | 0.17 ± 0.02 | N.D. |
| Polyunsaturated fatty acids (PUFA) | |||
| γ-Linolenic acid (C18:3n6) |  | 0.44 ± 0.03 | 1.41 ± 0.01 |
| Linoleic acid (C18:2n6c) |  | 0.49 ± 0.01 | 12.58 ± 0.01 |
| Arachidonic acid (C20:4n6) |  | 10.27 ± 0.02 | 5.26 ± 0.03 |
| Eicosapentaenoic acid (C20:5n3) |  | 1.33 ± 0.02 | 2.61 ± 0.01 |
| Dihomo-gamma-linolenic acid (C20:3n6) |  | 1.45 ± 0.04 | 0.51 ± 0.01 |
| Eicosadienoic acid (C20:2n6) |  | 0.61 ± 0.01 | 0.49 ± 0.01 |
| Docosahexaenoic acid (C22:6n3) |  | 0.95 ± 0.04 | 0.20 ± 0.00 |
| Indexes | Anomoeoneis Sp. AARL D039 | Rhopalodia Sp. AARL D020 |
|---|---|---|
| PS | 0.37 ± 0.02 | 0.56 ± 0.03 |
| IA | 0.68 ± 0.01 | 0.94 ± 0.03 |
| IT | 1.15 ± 0.03 | 1.11 ± 0.01 |
| h/H | 0.57 ± 0.02 | 0.82 ± 0.01 |
| HPI | 1.46 ± 0.02 | 1.06 ± 0.03 |
| UI | 104.01 ± 0.01 | 102.72 ± 0.20 |
| SED | 2.28 ± 0.01 | 2.81 ± 0.01 |
| TFA | 2.26 ± 0.03 | 3.14 ± 0.03 |
| Compounds | Sources | AutoDock Binding Free Energy, ∆G (kcal/mol) |
|---|---|---|
| Enalaprilat | Commercial drug | −14.15 |
| Hexanoic acid | Rho | −8.22 |
| Decanoic acid | Rho | −9.02 |
| Dodecanoic acid | Rho | −9.72 |
| cis-9 Tetradecenoic acid | Rho, Ano | −10.06 |
| Tetradecanoic acid | Rho, Ano | −10.18 |
| Pentadecanoic acid | Rho, Ano | −10.1 |
| (9Z)-hexadec-9-enoic acid | Rho, Ano | −10.54 |
| Hexadecanoic acid | Rho, Ano | −10.17 |
| cis-10 Heptadecenoic acid | Rho, Ano | −10.5 |
| Heptadecanoic acid | Rho, Ano | −10.34 |
| cis-6,9,12-octadecatrienoic acid | Rho, Ano | −11.37 |
| cis-9,12-octadecadienoic acid | Rho, Ano | −11.21 |
| (E)-9-octadecenoic acid | Rho, Ano | −10.99 |
| cis-9-octadecenoic acid | Rho, Ano | −10.8 |
| Octadecanoic acid | Rho, Ano | −10.44 |
| cis-5,8,11,14-eicosatetraenoic acid | Rho, Ano | −11.72 (3),* |
| cis-5,8,11,14,17-eicosapentaenoic acid | Rho, Ano | −11.78 (2),* |
| cis-8,11,14-eicosatrienoic acid | Rho, Ano | −11.53 |
| cis-11,14-eicosadienoic acid | Rho, Ano | −11.04 |
| cis-4,7,10,13,16,19-docosahexaenoic acid | Rho, Ano | −12.37 (1),* |
| cis-13-docosenoic acid | Ano | −11.57 |
| Docosanoic acid | Ano | −10.71 |
| cis-15-tetracosenoic acid | Ano | −11.23 |
| Tetracosanoic acid | Ano | −11.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pekkoh, J.; Phinyo, K.; Thurakit, T.; Lomakool, S.; Duangjan, K.; Ruangrit, K.; Pumas, C.; Jiranusornkul, S.; Yooin, W.; Cheirsilp, B.; et al. Lipid Profile, Antioxidant and Antihypertensive Activity, and Computational Molecular Docking of Diatom Fatty Acids as ACE Inhibitors. Antioxidants 2022, 11, 186. https://doi.org/10.3390/antiox11020186
Pekkoh J, Phinyo K, Thurakit T, Lomakool S, Duangjan K, Ruangrit K, Pumas C, Jiranusornkul S, Yooin W, Cheirsilp B, et al. Lipid Profile, Antioxidant and Antihypertensive Activity, and Computational Molecular Docking of Diatom Fatty Acids as ACE Inhibitors. Antioxidants. 2022; 11(2):186. https://doi.org/10.3390/antiox11020186
Chicago/Turabian StylePekkoh, Jeeraporn, Kittiya Phinyo, Theera Thurakit, Sureeporn Lomakool, Kritsana Duangjan, Khomsan Ruangrit, Chayakorn Pumas, Supat Jiranusornkul, Wipawadee Yooin, Benjamas Cheirsilp, and et al. 2022. "Lipid Profile, Antioxidant and Antihypertensive Activity, and Computational Molecular Docking of Diatom Fatty Acids as ACE Inhibitors" Antioxidants 11, no. 2: 186. https://doi.org/10.3390/antiox11020186
APA StylePekkoh, J., Phinyo, K., Thurakit, T., Lomakool, S., Duangjan, K., Ruangrit, K., Pumas, C., Jiranusornkul, S., Yooin, W., Cheirsilp, B., Pathom-aree, W., & Srinuanpan, S. (2022). Lipid Profile, Antioxidant and Antihypertensive Activity, and Computational Molecular Docking of Diatom Fatty Acids as ACE Inhibitors. Antioxidants, 11(2), 186. https://doi.org/10.3390/antiox11020186