Antioxidant and Anti-Inflammatory Activity of Combined Phycocyanin and Palmitoylethanolamide in Human Lung and Prostate Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Human Recombinant COX-2 Inhibitor Screening Assay
2.3. Cytokines Quantification
2.4. Measurement of Intracellular ROS Production
2.5. Toxicity Assay (LDH Release)
2.6. Measurement of Total Glutathione (GSH)
2.7. Real-Time Polymerase Chain Reaction (qRT-C-PCR)
2.8. Statistical Analysis
3. Results
3.1. PC and PEA Inhibit COX-2 Activity
3.2. PC and PEA Protect Cells from Oxidative Stress and Damage
3.3. PC and PEA Enhance the Antioxidant Defenses of the Cells
3.4. The Anti-Inflammatory Activity of PC and PEA Is Enhanced by the Combined Treatment
3.5. PC and PEA Decrease the Respiratory Burst Triggered by the Inflammatory Stimulus
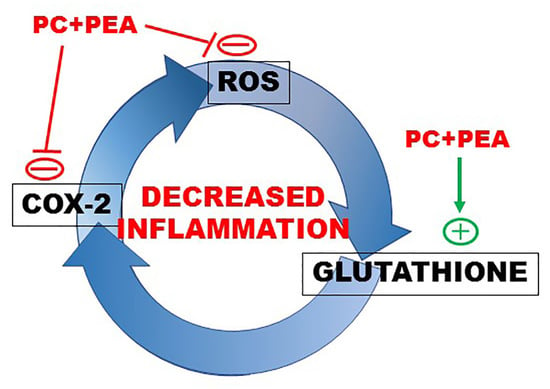
4. Discussion
- The direct inhibitory activity exerted by PEA on COX-2.
- The potentiation of antioxidant and anti-inflammatory activity exerted by the combination PC and PEA, which is due to a synergy in inhibiting COX-2 and consequent ROS production.
- The induction of glutathione synthesis by the combined treatment.
- The inhibition exerted by the two molecules on the respiratory burst triggered by bacterial or viral stimuli.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fioranelli, M.; Roccia, M.G.; Flavin, D.; Cota, L. Regulation of Inflammatory Reaction in Health and Disease. Int. J. Mol. Sci. 2021, 22, 5277. [Google Scholar] [CrossRef]
- Zhang, J.-M.; An, J. Cytokines, Inflammation and Pain. Int. Anesth. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chovatiya, R.; Medzhitov, R. Stress, Inflammation, and Defense of Homeostasis. Mol. Cell 2014, 54, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Martorana, F.; Guidotti, G.; Brambilla, L.; Rossi, D. Withaferin A Inhibits Nuclear Factor-ΚB-Dependent Pro-Inflammatory and Stress Response Pathways in the Astrocytes. Neural Plast. 2015, 2015, 381964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, S.H.; Hwang, D. Murine TOLL-like Receptor 4 Confers Lipopolysaccharide Responsiveness as Determined by Activation of NF Kappa B and Expression of the Inducible Cyclooxygenase. J. Biol. Chem. 2000, 275, 34035–34040. [Google Scholar] [CrossRef] [Green Version]
- Youn, H.-S.; Lim, H.J.; Choi, Y.J.; Lee, J.Y.; Lee, M.-Y.; Ryu, J.-H. Selenium Suppresses the Activation of Transcription Factor NF-Kappa B and IRF3 Induced by TLR3 or TLR4 Agonists. Int. Immunopharmacol. 2008, 8, 495–501. [Google Scholar] [CrossRef]
- Chen, C.-C. Signal Transduction Pathways of Inflammatory Gene Expressions and Therapeutic Implications. Curr. Pharm. Des. 2006, 12, 3497–3508. [Google Scholar] [CrossRef]
- Feng, L.; Xia, Y.; Garcia, G.E.; Hwang, D.; Wilson, C.B. Involvement of Reactive Oxygen Intermediates in Cyclooxygenase-2 Expression Induced by Interleukin-1, Tumor Necrosis Factor-Alpha, and Lipopolysaccharide. J. Clin. Investig. 1995, 95, 1669–1675. [Google Scholar] [CrossRef] [Green Version]
- Sancho, P.; Martín-Sanz, P.; Fabregat, I. Reciprocal Regulation of NADPH Oxidases and the Cyclooxygenase-2 Pathway. Free Radic. Biol. Med. 2011, 51, 1789–1798. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxidative Med. Cell. Longev. 2019, 2019, e6175804. [Google Scholar] [CrossRef]
- Chatterjee, S. Chapter Two—Oxidative Stress, Inflammation, and Disease. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 35–58. ISBN 978-0-12-803269-5. [Google Scholar]
- Koeberle, S.C.; Gollowitzer, A.; Laoukili, J.; Kranenburg, O.; Werz, O.; Koeberle, A.; Kipp, A.P. Distinct and Overlapping Functions of Glutathione Peroxidases 1 and 2 in Limiting NF-ΚB-Driven Inflammation through Redox-Active Mechanisms. Redox Biol. 2019, 28, 101388. [Google Scholar] [CrossRef]
- Banning, A.; Florian, S.; Deubel, S.; Thalmann, S.; Müller-Schmehl, K.; Jacobasch, G.; Brigelius-Flohé, R. GPx2 Counteracts PGE2 Production by Dampening COX-2 and MPGES-1 Expression in Human Colon Cancer Cells. Antioxid. Redox Signal. 2008, 10, 1491–1500. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Kefayat, A.; Ghahremani, F.; Safavi, A.; Hajiaghababa, A.; Moshtaghian, J. C-Phycocyanin: A Natural Product with Radiosensitizing Property for Enhancement of Colon Cancer Radiation Therapy Efficacy through Inhibition of COX-2 Expression. Sci. Rep. 2019, 9, 19161. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The Antioxidant, Immunomodulatory, and Anti-Inflammatory Activities of Spirulina: An Overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Fernández-Rojas, B.; Hernández-Juárez, J.; Pedraza-Chaverri, J. Nutraceutical Properties of Phycocyanin. J. Funct. Foods 2014, 11, 375–392. [Google Scholar] [CrossRef]
- Reddy, C.M.; Bhat, V.B.; Kiranmai, G.; Reddy, M.N.; Reddanna, P.; Madyastha, K.M. Selective Inhibition of Cyclooxygenase-2 by C-Phycocyanin, a Biliprotein from Spirulina Platensis. Biochem. Biophys. Res. Commun. 2000, 277, 599–603. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Yu, Y.; Li, W.; Liu, B.; Jiao, X.; Song, X.; Lv, C.; Qin, S. Phycocyanin Attenuates Pulmonary Fibrosis via the TLR2-MyD88-NF-ΚB Signaling Pathway. Sci. Rep. 2017, 7, 5843. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhang, J.; Yan, Y.; Chi, M.; Chen, W.; Sun, P.; Qin, S. The Protective Effect of C-Phycocyanin on Paraquat-Induced Acute Lung Injury in Rats. Environ. Toxicol. Pharm. 2011, 32, 168–174. [Google Scholar] [CrossRef]
- Leung, P.-O.; Lee, H.-H.; Kung, Y.-C.; Tsai, M.-F.; Chou, T.-C. Therapeutic Effect of C-Phycocyanin Extracted from Blue Green Algae in a Rat Model of Acute Lung Injury Induced by Lipopolysaccharide. Evid.-Based Complement. Altern. Med. 2013, 2013, 916590. [Google Scholar] [CrossRef] [Green Version]
- Alhouayek, M.; Muccioli, G.G. Harnessing the Anti-Inflammatory Potential of Palmitoylethanolamide. Drug Discov. Today 2014, 19, 1632–1639. [Google Scholar] [CrossRef]
- Peritore, A.F.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Crupi, R.; Genovese, T.; Impellizzeri, D.; Cuzzocrea, S.; et al. Ultramicronized Palmitoylethanolamide and Paracetamol, a New Association to Relieve Hyperalgesia and Pain in a Sciatic Nerve Injury Model in Rat. Int. J. Mol. Sci. 2020, 21, 3509. [Google Scholar] [CrossRef]
- Sohn, J.H.; Han, K.-L.; Lee, S.-H.; Hwang, J.-K. Protective Effects of Panduratin A against Oxidative Damage of Tert-Butylhydroperoxide in Human HepG2 Cells. Biol. Pharm. Bull. 2005, 28, 1083–1086. [Google Scholar] [CrossRef] [Green Version]
- Ricca, C.; Aillon, A.; Bergandi, L.; Alotto, D.; Castagnoli, C.; Silvagno, F. Vitamin D Receptor Is Necessary for Mitochondrial Function and Cell Health. Int. J. Mol. Sci. 2018, 19, 1672. [Google Scholar] [CrossRef] [Green Version]
- Destefanis, M.; Viano, M.; Leo, C.; Gervino, G.; Ponzetto, A.; Silvagno, F. Extremely Low Frequency Electromagnetic Fields Affect Proliferation and Mitochondrial Activity of Human Cancer Cell Lines. Int. J. Radiat. Biol. 2015, 91, 964–972. [Google Scholar] [CrossRef] [Green Version]
- Soderholm, A.T.; Barnett, T.C.; Korn, O.; Rivera-Hernandez, T.; Seymour, L.M.; Schulz, B.L.; Nizet, V.; Wells, C.A.; Sweet, M.J.; Walker, M.J. Group A Streptococcus M1T1 Intracellular Infection of Primary Tonsil Epithelial Cells Dampens Levels of Secreted IL-8 Through the Action of SpyCEP. Front. Cell Infect. Microbiol. 2018, 8, 160. [Google Scholar] [CrossRef] [Green Version]
- Steensberg, A.; Febbraio, M.A.; Osada, T.; Schjerling, P.; van Hall, G.; Saltin, B.; Pedersen, B.K. Interleukin-6 Production in Contracting Human Skeletal Muscle Is Influenced by Pre-Exercise Muscle Glycogen Content. J. Physiol. 2001, 537, 633–639. [Google Scholar] [CrossRef]
- Subhan, F.; Kang, H.Y.; Lim, Y.; Ikram, M.; Baek, S.-Y.; Jin, S.; Jeong, Y.H.; Kwak, J.Y.; Yoon, S. Fish Scale Collagen Peptides Protect against CoCl2/TNF-α-Induced Cytotoxicity and Inflammation via Inhibition of ROS, MAPK, and NF-ΚB Pathways in HaCaT Cells. Oxidative Med. Cell. Longev. 2017, 2017, 9703609. [Google Scholar] [CrossRef] [Green Version]
- Bergandi, L.; Lucia, U.; Grisolia, G.; Granata, R.; Gesmundo, I.; Ponzetto, A.; Paolucci, E.; Borchiellini, R.; Ghigo, E.; Silvagno, F. The Extremely Low Frequency Electromagnetic Stimulation Selective for Cancer Cells Elicits Growth Arrest through a Metabolic Shift. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1389–1397. [Google Scholar] [CrossRef]
- Jin, Y.; Huang, Z.; Li, L.; Yang, Y.; Wang, C.; Wang, Z.; Ji, L. Quercetin Attenuates Toosendanin-Induced Hepatotoxicity through Inducing the Nrf2/GCL/GSH Antioxidant Signaling Pathway. Acta Pharm. Sin. 2019, 40, 75–85. [Google Scholar] [CrossRef]
- Hao, S.; Li, S.; Wang, J.; Yan, Y.; Ai, X.; Zhang, J.; Ren, Y.; Wu, T.; Liu, L.; Wang, C. Phycocyanin Exerts Anti-Proliferative Effects through Down-Regulating TIRAP/NF-ΚB Activity in Human Non-Small Cell Lung Cancer Cells. Cells 2019, 8, 588. [Google Scholar] [CrossRef] [Green Version]
- Peritore, A.F.; D’Amico, R.; Siracusa, R.; Cordaro, M.; Fusco, R.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Di Paola, R.; Cuzzocrea, S.; et al. Management of Acute Lung Injury: Palmitoylethanolamide as a New Approach. Int. J. Mol. Sci. 2021, 22, 5533. [Google Scholar] [CrossRef]
- Cordaro, M.; Impellizzeri, D.; Siracusa, R.; Gugliandolo, E.; Fusco, R.; Inferrera, A.; Esposito, E.; Di Paola, R.; Cuzzocrea, S. Effects of a Co-Micronized Composite Containing Palmitoylethanolamide and Polydatin in an Experimental Model of Benign Prostatic Hyperplasia. Toxicol. Appl. Pharm. 2017, 329, 231–240. [Google Scholar] [CrossRef]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of Double-Stranded RNA and Activation of NF-KappaB by Toll-like Receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
- DeLeve, L.D.; Kaplowitz, N. Glutathione Metabolism and Its Role in Hepatotoxicity. Pharmacol. Ther. 1991, 52, 287–305. [Google Scholar] [CrossRef]
- Zheng, J.-N.; Zhuo, J.-Y.; Nie, J.; Liu, Y.-L.; Chen, B.-Y.; Wu, A.-Z.; Li, Y.-C. Phenylethanoid Glycosides From Callicarpa Kwangtungensis Chun Attenuate TNF-α-Induced Cell Damage by Inhibiting NF-ΚB Pathway and Enhancing Nrf2 Pathway in A549 Cells. Front. Pharmacol. 2021, 12, 693983. [Google Scholar] [CrossRef]
- Reimer, T.; Brcic, M.; Schweizer, M.; Jungi, T.W. Poly(I:C) and LPS Induce Distinct IRF3 and NF-KappaB Signaling during Type-I IFN and TNF Responses in Human Macrophages. J. Leukoc. Biol. 2008, 83, 1249–1257. [Google Scholar] [CrossRef]
- West, A.P.; Brodsky, I.E.; Rahner, C.; Woo, D.K.; Erdjument-Bromage, H.; Tempst, P.; Walsh, M.C.; Choi, Y.; Shadel, G.S.; Ghosh, S. TLR Signalling Augments Macrophage Bactericidal Activity through Mitochondrial ROS. Nature 2011, 472, 476–480. [Google Scholar] [CrossRef] [Green Version]
- Zarghi, A.; Arfaei, S. Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships. Iran. J. Pharm. Res. 2011, 10, 655–683. [Google Scholar]
- Gabrielsson, L.; Gouveia-Figueira, S.; Häggström, J.; Alhouayek, M.; Fowler, C.J. The Anti-inflammatory Compound Palmitoylethanolamide Inhibits Prostaglandin and Hydroxyeicosatetraenoic Acid Production by a Macrophage Cell Line. Pharmacol. Res. Perspect. 2017, 5, e00300. [Google Scholar] [CrossRef]
- Talley, J.J.; Brown, D.L.; Carter, J.S.; Graneto, M.J.; Koboldt, C.M.; Masferrer, J.L.; Perkins, W.E.; Rogers, R.S.; Shaffer, A.F.; Zhang, Y.Y.; et al. 4-[5-Methyl-3-Phenylisoxazol-4-Yl]- Benzenesulfonamide, Valdecoxib: A Potent and Selective Inhibitor of COX-2. J. Med. Chem. 2000, 43, 775–777. [Google Scholar] [CrossRef]
- Chan, C.C.; Boyce, S.; Brideau, C.; Charleson, S.; Cromlish, W.; Ethier, D.; Evans, J.; Ford-Hutchinson, A.W.; Forrest, M.J.; Gauthier, J.Y.; et al. Rofecoxib [Vioxx, MK-0966; 4-(4′-Methylsulfonylphenyl)-3-Phenyl-2-(5H)-Furanone]: A Potent and Orally Active Cyclooxygenase-2 Inhibitor. Pharmacological and Biochemical Profiles. J. Pharmacol. Exp. Ther. 1999, 290, 551–560. [Google Scholar]
- Yoshino, T.; Kimoto, A.; Kobayashi, S.; Noguchi, M.; Fukunaga, M.; Hayashi, A.; Miyata, K.; Sasamata, M. Pharmacological Profile of Celecoxib, a Specific Cyclooxygenase-2 Inhibitor. Arzneimittelforschung 2005, 55, 394–402. [Google Scholar] [CrossRef]
- Arora, M.; Choudhary, S.; Singh, P.K.; Sapra, B.; Silakari, O. Structural Investigation on the Selective COX-2 Inhibitors Mediated Cardiotoxicity: A Review. Life Sci. 2020, 251, 117631. [Google Scholar] [CrossRef]
- Choudhary, S.; Silakari, O. Butterfly Structure: A Scaffold of Therapeutic Importance. Future Med. Chem. 2019, 12, 179–182. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.-J.; Lee, H.; Kim, G.-Y.; Choi, Y.H. The Regulation of the TLR4/NF-ΚB and Nrf2/HO-1 Signaling Pathways Is Involved in the Inhibition of Lipopolysaccharide-Induced Inflammation and Oxidative Reactions by Morroniside in RAW 264.7 Macrophages. Arch. Biochem. Biophys. 2021, 706, 108926. [Google Scholar] [CrossRef]
- Rubattu, S.; Mennuni, S.; Testa, M.; Mennuni, M.; Pierelli, G.; Pagliaro, B.; Gabriele, E.; Coluccia, R.; Autore, C.; Volpe, M. Pathogenesis of Chronic Cardiorenal Syndrome: Is There a Role for Oxidative Stress? Int. J. Mol. Sci. 2013, 14, 23011–23032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minghetti, L. Cyclooxygenase-2 (COX-2) in Inflammatory and Degenerative Brain Diseases. J. Neuropathol. Exp. Neurol. 2004, 63, 901–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Xu, X.; Wang, L.; Su, L.; Gu, Q.; Wei, F.; Liu, K. Inhibitory Effect of a Novel Peptide, H-RN, on Keratitis Induced by LPS or Poly(I:C) In Vitro and In Vivo via Suppressing NF-ΚB and MAPK Activation. J. Transl. Med. 2017, 15, 20. [Google Scholar] [CrossRef] [Green Version]
- Uberti, F.; Ruga, S.; Farghali, M.; Galla, R.; Molinari, C. A Combination of α-Lipoic Acid (ALA) and Palmitoylethanolamide (PEA) Blocks Endotoxin-Induced Oxidative Stress and Cytokine Storm: A Possible Intervention for COVID-19. J. Diet. Suppl. 2021. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.S.; Steinauer, K.K.; Hornung, B.; Irish, J.M.; Lecane, P.; Birrell, G.W.; Peehl, D.M.; Knox, S.J. Role of Glutathione Depletion and Reactive Oxygen Species Generation in Apoptotic Signaling in a Human B Lymphoma Cell Line. Cell Death Differ. 2002, 9, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-H.; Lee, S.-G.; Shin, J.-S.; Lee, H.-Y.; Yoon, K.; Ji, Y.W.; Jang, D.S.; Lee, K.-T. P-Coumaroyl Anthocyanin Mixture Isolated from Tuber Epidermis of Solanum Tuberosum Attenuates Reactive Oxygen Species and Pro-Inflammatory Mediators by Suppressing NF-ΚB and STAT1/3 Signaling in LPS-Induced RAW264.7 Macrophages. Biol. Pharm. Bull. 2017, 40, 1894–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myhrstad, M.C.W.; Carlsen, H.; Nordström, O.; Blomhoff, R.; Moskaug, J.Ø. Flavonoids Increase the Intracellular Glutathione Level by Transactivation of the Gamma-Glutamylcysteine Synthetase Catalytical Subunit Promoter. Free Radic. Biol. Med. 2002, 32, 386–393. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Lee, H.; Rangasamy, T.; Reddy, S.P.; Yamamoto, M.; Kensler, T.W.; Biswal, S. Nrf2 Is a Critical Regulator of the Innate Immune Response and Survival during Experimental Sepsis. J. Clin. Investig. 2006, 116, 984–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maack, C.; Böhm, M. Targeting Mitochondrial Oxidative Stress in Heart Failure Throttling the Afterburner. J. Am. Coll. Cardiol. 2011, 58, 83–86. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Liu, Q.; Han, J.; Feng, J.; Guo, T.; Li, Z.; Min, F.; Jin, R.; Peng, X. N-Acetylcysteine Inhibits Patulin-Induced Apoptosis by Affecting ROS-Mediated Oxidative Damage Pathway. Toxins 2021, 13, 595. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yoon, J.-H.; Won, H.-J.; Ji, H.-S.; Yuk, H.J.; Park, K.H.; Park, H.-Y.; Jeong, T.-S. Isotrifoliol Inhibits Pro-Inflammatory Mediators by Suppression of TLR/NF-ΚB and TLR/MAPK Signaling in LPS-Induced RAW264.7 Cells. Int. Immunopharmacol. 2017, 45, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Lee, E.K.; Gong, S.Y.; Sohng, J.K.; Kang, Y.J.; Jung, H.J. Sparassis Crispa Exerts Anti-Inflammatory Activity via Suppression of TLR-Mediated NF-ΚB and MAPK Signaling Pathways in LPS-Induced RAW264.7 Macrophage Cells. J. Ethnopharmacol. 2019, 231, 10–18. [Google Scholar] [CrossRef]
- Elmasry, S.A.; Al-Azzawi, M.A.; Ghoneim, A.H.; Nasr, M.Y.; AboZaid, M.M.N. Role of Oxidant–Antioxidant Imbalance in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Egypt. J. Chest Dis. Tuberc. 2015, 64, 813–820. [Google Scholar] [CrossRef] [Green Version]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [Green Version]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-Induced Cancer: Crosstalk between Tumours, Immune Cells and Microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and Chemokines: At the Crossroads of Cell Signalling and Inflammatory Disease. Biochim. Et Biophys. Acta Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-H.; Park, J.-S.; Kim, D.-H.; Kang, J.L.; Kim, H.-S. Anti-Inflammatory Mechanism of Lonchocarpine in LPS- or Poly(I:C)-Induced Neuroinflammation. Pharmacol. Res. 2017, 119, 431–442. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergandi, L.; Apprato, G.; Silvagno, F. Antioxidant and Anti-Inflammatory Activity of Combined Phycocyanin and Palmitoylethanolamide in Human Lung and Prostate Epithelial Cells. Antioxidants 2022, 11, 201. https://doi.org/10.3390/antiox11020201
Bergandi L, Apprato G, Silvagno F. Antioxidant and Anti-Inflammatory Activity of Combined Phycocyanin and Palmitoylethanolamide in Human Lung and Prostate Epithelial Cells. Antioxidants. 2022; 11(2):201. https://doi.org/10.3390/antiox11020201
Chicago/Turabian StyleBergandi, Loredana, Giulia Apprato, and Francesca Silvagno. 2022. "Antioxidant and Anti-Inflammatory Activity of Combined Phycocyanin and Palmitoylethanolamide in Human Lung and Prostate Epithelial Cells" Antioxidants 11, no. 2: 201. https://doi.org/10.3390/antiox11020201
APA StyleBergandi, L., Apprato, G., & Silvagno, F. (2022). Antioxidant and Anti-Inflammatory Activity of Combined Phycocyanin and Palmitoylethanolamide in Human Lung and Prostate Epithelial Cells. Antioxidants, 11(2), 201. https://doi.org/10.3390/antiox11020201