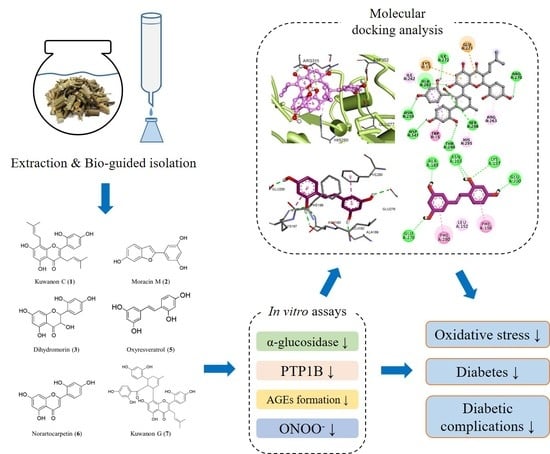
Inhibition Mechanism of Components Isolated from Morus alba Branches on Diabetes and Diabetic Complications via Experimental and Molecular Docking Analyses
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Chemicals and Reagents
2.3. Plant Material
2.4. Extraction, Fractionation, and Isolation
2.4.1. Kuwanon C (1)
2.4.2. Moracin M (2)
2.4.3. Dihydromorin (3)

2.4.4. β-Sitosterol Glucoside (4)
2.4.5. Oxyresveratrol (5)
2.4.6. Norartocarpetin (6)
2.4.7. Kuwanon G (7)
2.4.8. Kaempferol 7-O-β-d-glucopyranoside (8)
2.5. In Vitro Assay for α-Glucosidase Inhibitory Activity
2.6. In Vitro Assay for PTP1B Inhibitory Activity
2.7. In Vitro Assay for Inhibition of AGE Formation
2.8. In Vitro Assay for Peroxynitrite Scavenging Activity
2.9. Kinetic Study for α-Glucosidase and PTP1B Inhibition
2.10. Molecular Docking Analysis for α-Glucosidase and PTP1B Inhibition
3. Results
3.1. Inhibitory Activity of the Methanol Extract and Its Fractions on α-Glucosidase, PTP1B, AGEs, and Peroxynitrite
3.2. Inhibitory Activity of Isolated Compounds on α-Glucosidase and PTP1B
3.3. Inhibitory Activity of Isolated Compounds against AGE Formation and Peroxynitrite
| Compounds | α-Glucosidase | PTP1B | AGEs | ONOO− | ||||
|---|---|---|---|---|---|---|---|---|
| IC50 (μM) 1 | Inhibition Mode 2 | Ki (μM) 3 | IC50 (μM) 1 | Inhibition Mode 2 | Ki (μM) 3 | IC50 (μM) 1 | ||
| Kuwanon C (1) | 14.75 ± 0.88 | Mixed | 6.85 | 41.43 ± 1.64 | Non-competitive | 39.43 | >100 b | 12.92 ± 0.68 |
| Moracin M (2) | 32.43 ± 1.65 | Mixed | 3.32 | 333.1 ± 20.53 | - | - | 2.10 ± 0.22 | 1.08 ± 0.04 |
| Dihydromorin (3) | 47.35 ± 2.25 | Mixed | 10.22 | 180.2 ± 0.77 | - | - | 117.5 ± 7.89 | 2.26 ± 0.12 |
| Oxyresveratrol (5) | 1.86 ± 0.20 | Mixed | 1.14 | 2.85 ± 0.30 | Non-competitive | 2.16 | 7.56 ± 0.15 | 2.37 ± 0.21 |
| Norartocarpetin (6) | 31.95 ± 1.72 | Mixed | 19.90 | >100 5 | - | - | 77.29 ± 9.58 | 3.01 ± 0.15 |
| Kuwanon G (7) | 1.44 ± 0.11 | Mixed | 2.03 | 16.17 ± 0.29 | Mixed | 12.41 | 69.07 ± 1.49 | 6.35 ± 0.36 |
| Acarbose 4 | 350.9 ± 17.94 | - | - | - | - | - | - | - |
| Ursolic acid 4 | - | - | - | 16.48 ± 2.07 | - | - | - | - |
| Aminoguanidine 4 | - | - | 890.3 ± 70.16 | - | ||||
| l-Penicillamine 4 | - | - | - | 6.69 ± 0.52 | ||||
3.4. Enzyme Kinetic Study of Compounds in α-Glucosidase and PTP1B Inhibition
3.5. Molecular Docking Analysis for α-Glucosidase Inhibition
3.6. Molecular Docking Analysis for PTP1B Inhibition


| Compounds | Binding Energy 1 | Number of H-Bonds | H-Bond Interacting Residues | Hydrophobic Interacting Residues | Electrostatic Interacting Residues |
|---|---|---|---|---|---|
| Kuwanon C (1) | −7.54 | 3 | Glu200, Gly277, Ala189 | Phe196(Pi-Pi Stacked), Phe280(Pi-Pi Stacked, Pi-Alkyl), Lys197(Alkyl), Leu192(Pi-Alkyl) | |
| Oxyresveratrol (5) | −6.98 | 6 | Asn193, Lys197, GLu200, Glu276, Ala189 | Phe280(Pi-Pi Stacked), Phe196(Amide-Pi Stacked), Leu192(Pi-Alkyl) | |
| Kuwanon G (7) | −6.26 | 7 | Ser216, Gln266, Asp48, Met258, Gln262, Tyr46 | Tyr46(Pi-Pi Stacked), Ala217(Alkyl), Ile219(Alkyl), Trp179(Pi-Alkyl), Val49(Pi-Alkyl), Arg221(Pi-Alkyl) | Arg221(Pi-Cation) |
| −7.11 | 5 | Asn193, Gly277, Phe280, Glu200 | Phe196(Pi-Pi Stacked, Pi-Pi T-shaped, Pi-Alkyl), Phe280(Pi-Pi Stacked, Pi-Pi T-shaped, Pi-Alkyl), Ile281(Alkyl), Leu192(Pi-Alkyl) | Lys197(Pi-Cation) | |
| Compound A 2 | −10.2 | 10 | Ser216, Ala217, Gly218, Ile219, Gly220,Arg221, Arg254, Asp48 | Ala217(Pi-Sigma, Pi-Alkyl), Tyr46(Pi-Sigma, Pi-Pi Stacked), Ala27(Pi-Pi Stacked) | |
| Compound B 3 | −9.08 | 2 | Asn193, Glu276 | Phe196(Pi-Sigma, Pi-Alkyl), Phe280(Pi-Pi Stacked, Pi-Pi T-shaped, Pi-Alkyl), Ile281(Alkyl), Leu192(Pi-Alkyl), Ala189(Pi-Alkyl) |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, S.Y.; Wong, J.L.M.; Sim, Y.J.; Wong, S.S.; Elhassan, S.A.M.; Tan, S.H.; Lim, G.P.L.; Tay, N.W.R.; Annan, N.C.; Bhattamisra, S.K. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 364–372. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Mohammad, G. Epigenetic modifications in diabetes. Metabolism 2022, 126, 154920. [Google Scholar] [CrossRef] [PubMed]
- Ndjaboue, R.; Farhat, I.; Ferlatte, C.-A.; Ngueta, G.; Guay, D.; Delorme, S.; Ivers, N.; Shah, B.R.; Straus, S.; Yu, C. Predictive models of diabetes complications: Protocol for a scoping review. Syst. Rev. 2020, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P. α-Glucosidase inhibitors and their use in clinical practice. Arch. Med. Sci. 2012, 8, 899. [Google Scholar] [CrossRef]
- Fowler, M.J. Diabetes treatment, part 2: Oral agents for glycemic management. Clin. Diavetes 2007, 25, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure–activity relationship study. J. Enzym. Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef] [Green Version]
- Mertes, G. Safety and efficacy of acarbose in the treatment of type 2 diabetes: Data from a 5-year surveillance study. Diabetes Res. Clin. Pract. 2001, 52, 193–204. [Google Scholar] [CrossRef]
- Goldstein, B.J. Protein-tyrosine phosphatases: Emerging targets for therapeutic intervention in type 2 diabetes and related states of insulin resistance. J. Clin. Endocrinol. Metab. 2002, 87, 2474–2480. [Google Scholar] [CrossRef]
- Zhang, B.W.; Xing, Y.; Wen, C.; Yu, X.X.; Sun, W.L.; Xiu, Z.L.; Dong, Y.S. Pentacyclic triterpenes as α-glucosidase and α-amylase inhibitors: Structure-activity relationships and the synergism with acarbose. Bioorganic Med. Chem. Lett. 2017, 27, 5065–5070. [Google Scholar] [CrossRef]
- Goh, S.Y.; Cooper, M.E. The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef] [Green Version]
- Thornalley, P.J.; Yurek-George, A.; Argirov, O.K. Kinetics and mechanism of the reaction of aminoguanidine with the α-oxoaldehydes glyoxal, methylglyoxal, and 3-deoxyglucosone under physiological conditions. Biochem. Pharmacol. 2000, 60, 55–65. [Google Scholar] [CrossRef]
- Freedman, B.I.; Wuerth, J.P.; Cartwright, K.; Bain, R.P.; Dippe, S.; Hershon, K.; Mooradian, A.D.; Spinowitz, B.S. Design and baseline characteristics for the aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II). Control. Clin. Trials 1999, 20, 493–510. [Google Scholar] [CrossRef]
- Gardner, C.D.; Eguchi, S.; Reynolds, C.M.; Eguchi, K.; Frank, G.D.; Motley, E.D. Hydrogen peroxide inhibits insulin signaling in vascular smooth muscle cells. Exp. Biol. Med. 2003, 228, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Nagai, R.; Unno, Y.; Hayashi, M.C.; Masuda, S.; Hayase, F.; Kinae, N.; Horiuchi, S. Peroxynitrite induces formation of Nε-(carboxymethyl) lysine by the cleavage of Amadori product and generation of glucosone and glyoxal from glucose: Novel pathways for protein modification by peroxynitrite. Diabetes 2002, 51, 2833–2839. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Li, H.; Zeng, J.; Huang, K. Effects of peroxynitrite-induced protein tyrosine nitration on insulin-stimulated tyrosine phosphorylation in HepG2 cells. Mol. Cell Biochem. 2009, 331, 49–57. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, K. Peroxynitrite mediates muscle insulin resistance in mice via nitration of IRβ/IRS-1 and Akt. Toxicol. Appl. Pharmacol. 2009, 241, 101–110. [Google Scholar] [CrossRef]
- Alam, M.M.; Meerza, D.; Naseem, I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014, 109, 8–14. [Google Scholar] [CrossRef]
- Zelová, H.; Hanáková, Z.; Čermáková, Z.; Šmejkal, K.; Dalĺ Acqua, S.; Babula, P.; Cvačka, J.; Hošek, J. Evaluation of anti-inflammatory activity of prenylated substances isolated from Morus alba and Morus nigra. J. Nat. Prod. 2014, 77, 1297–1303. [Google Scholar] [CrossRef]
- Choi, S.W.; Jang, Y.J.; Lee, Y.J.; Leem, H.H.; Kim, E.O. Analysis of functional constituents in mulberry (Morus alba L.) twigs by different cultivars, producing areas, and heat processings. Prev. Nutr. Food Sci. 2013, 18, 256. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Jin, B.; Shin, J.H.; Adisakwattana, S.; Kwon, O. Standardized Mori ramulus extract improves insulin secretion and insulin sensitivity in C57BLKS/J db/db mice and INS-1 cells. Biomed. Pharamcother. 2017, 92, 308–315. [Google Scholar] [CrossRef]
- Abbas, G.M.; Abdel Bar, F.M.; Baraka, H.N.; Gohar, A.A.; Lahloub, M.-F. A new antioxidant stilbene and other constituents from the stem bark of Morus nigra L. Nat. Prod. Res. 2014, 28, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Basnet, P.; KADoTA, S.; Terashima, S.; Shimizu, M.; Namba, T. Two new 2-arylbenzofuran derivatives from hypoglycemic activity-bearing fractions of Morus insignis. Chem. Pharm. Bull. 1993, 41, 1238–1243. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.P.; Cheng, K.W.; To, J.T.K.; Li, H.; Wang, M. Isolation of tyrosinase inhibitors from Artocarpus heterophyllus and use of its extract as antibrowning agent. Mol. Nutr. Food Res. 2008, 52, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Youn, U.J.; Chang, L.C. Chemical constituents of fermented noni (Morinda citrifolia) juice exudates and their biological activity. Nat. Prod. Sci. 2017, 23, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.B.; Yoon, J.; Lee, H.Y.; Choi, H.S.; Lee, H.B. Use of Compounds Isolated from Morus Bark. United States Patent US 20,140,018,552 A1, 26 March 2012. [Google Scholar]
- Ko, H.H.; Tsai, Y.T.; Yen, M.H.; Lin, C.C.; Liang, C.J.; Yang, T.H.; Lee, C.W.; Yen, F.L. Norartocarpetin from a folk medicine Artocarpus communis plays a melanogenesis inhibitor without cytotoxicity in B16F10 cell and skin irritation in mice. BMC Complement. Altern. Med. 2013, 13, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyệt, B.T.M.; Khanh, T.H.N. THE ISOLATED COMPOUNDS FROM THE TWIGS OF MORUS ALBA L. IN DONG THAP. Vietnam J. Sci. Technol. 2016, 54, 509. [Google Scholar] [CrossRef] [Green Version]
- Park, J.C.; Young, H.S.; Choi, J.S. Constituents of Cudrania tricuspidata in Korea. Yakhak Hoeji 1992, 36, 40–45. [Google Scholar]
- Ting, L.; Zhang, X.; Song, Y.; Liu, J. A microplate-based screening method for alpha-glucosidase inhibitors. Chin. J. Clin. Pharmacol. Therapeut. 2005, 10, 1128–1134. [Google Scholar]
- Cui, L.; Na, M.; Oh, H.; Bae, E.Y.; Jeong, D.G.; Ryu, S.E.; Kim, S.; Kim, B.Y.; Oh, W.K.; Ahn, J.S. Protein tyrosine phosphatase 1B inhibitors from Morus root bark. Bioorg. Med. Chem. Lett. 2006, 16, 1426–1429. [Google Scholar] [CrossRef]
- Vinson, J.A.; Howard Iii, T.B. Inhibition of protein glycation and advanced glycation end products by ascorbic acid and other vitamins and nutrients. J. Nutr. Biochem. 1996, 7, 659–663. [Google Scholar] [CrossRef]
- Kooy, N.W.; Royall, J.A.; Ischiropoulos, H.; Beckman, J.S. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic. Biol. Med. 1994, 16, 149–156. [Google Scholar] [CrossRef]
- Athel, C.B. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem. J. 1974, 137, 143–144. [Google Scholar]
- Dixon, M. The determination of enzyme inhibitor constants. Biochem. J. 1953, 55, 170. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Yamamoto, K.; Miyake, H.; Kusunoki, M.; Osaki, S. Crystal structures of isomaltase from Saccharomyces cerevisiae and in complex with its competitive inhibitor maltose. FEBS J. 2010, 277, 4205–4214. [Google Scholar] [CrossRef]
- Szczepankiewicz, B.G.; Liu, G.; Hajduk, P.J.; Abad-Zapatero, C.; Pei, Z.; Xin, Z.; Lubben, T.H.; Trevillyan, J.M.; Stashko, M.A.; Ballaron, S.J. Discovery of a potent, selective protein tyrosine phosphatase 1B inhibitor using a linked-fragment strategy. J. Am. Chem. Soc. 2003, 125, 4087–4096. [Google Scholar] [CrossRef]
- Wiesmann, C.; Barr, K.J.; Kung, J.; Zhu, J.; Erlanson, D.A.; Shen, W.; Fahr, B.J.; Zhong, M.; Taylor, L.; Randal, M. Allosteric inhibition of protein tyrosine phosphatase 1B. Nat. Struct. Mol. Biol. 2004, 11, 730. [Google Scholar] [CrossRef]
- Lahlou, M. The success of natural products in drug discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Li, Y.; Zhang, X.; Lei, Y.; Ding, W.; Zhao, X.; Wang, H.; Song, X.; Yao, Q.; Zhang, Y. Synthesis, α-glucosidase inhibitory and molecular docking studies of prenylated and geranylated flavones, isoflavones and chalcones. Bioorg. Med. Chem. Lett. 2015, 25, 4567–4571. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.T.; Nguyen, D.H.; Le, D.D.; Choi, J.S.; Min, B.S.; Woo, M.H. Protein tyrosine phosphatase 1B inhibitors from natural sources. Arch. Pharm. Res. 2018, 41, 130–161. [Google Scholar] [CrossRef] [PubMed]
- Mascarello, A.; Menegatti, A.C.O.; Calcaterra, A.; Martins, P.G.A.; Chiaradia-Delatorre, L.D.; D’Acquarica, I.; Ferrari, F.; Pau, V.; Sanna, A.; De Logu, A.; et al. Naturally occurring Diels-Alder-type adducts from Morus nigra as potent inhibitors of Mycobacterium tuberculosis protein tyrosine phosphatase B. Eur. J. Med. Chem. 2018, 144, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.K.; Bayly, C.I.; Gauthier, J.Y.; Li, C.S.; Therien, M.; Asante-Appiah, E.; Cromlish, W.; Boie, Y.; Forghani, F.; Desmarais, S. Structure based design of a series of potent and selective non peptidic PTP-1B inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Z.Y. PTP1B as a drug target: Recent developments in PTP1B inhibitor discovery. Drug Discov. Today 2007, 12, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Paudel, P.; Yu, T.; Seong, S.H.; Kuk, E.B.; Jung, H.A.; Choi, J.S. Protein tyrosine phosphatase 1B inhibition and glucose uptake potentials of mulberrofuran G, albanol B, and kuwanon G from root bark of Morus alba L. in insulin-resistant HepG2 cells: An in vitro and in silico study. Int. J. Mol. Sci. 2018, 19, 1542. [Google Scholar] [CrossRef] [Green Version]
- Yar, M.; Bajda, M.; Shahzadi, L.; Shahzad, S.A.; Ahmed, M.; Ashraf, M.; Alam, U.; Khan, I.U.; Khan, A.F. Novel synthesis of dihydropyrimidines for α-glucosidase inhibition to treat type 2 diabetes: In vitro biological evaluation and in silico docking. Bioorg. Chem. 2014, 54, 96–104. [Google Scholar] [CrossRef]
- Yamamoto, K.; Nakayama, A.; Yamamoto, Y.; Tabata, S. Val216 decides the substrate specificity of α-glucosidase in Saccharomyces cerevisiae. Eur. J. Biochem. 2004, 271, 3414–3420. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Sari, S.; Özel, A.; Barut, B. α-Glucosidase inhibitory effect of Potentilla astracanica and some isoflavones: Inhibition kinetics and mechanistic insights through in vitro and in silico studies. Int. J. Biol. Macromol. 2017, 105, 1062–1070. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Lu, S.; Huang, W.; Geng, L.; Shen, Q.; Zhang, J. The mechanism of allosteric inhibition of protein tyrosine phosphatase 1B. PLoS ONE 2014, 9, e97668. [Google Scholar] [CrossRef] [Green Version]
- Shinde, R.N.; Kumar, G.S.; Eqbal, S.; Sobhia, M.E. Screening and identification of potential PTP1B allosteric inhibitors using in silico and in vitro approaches. PLoS ONE 2018, 13, e0199020. [Google Scholar] [CrossRef] [PubMed]
- Barford, D.; Flint, A.J.; Tonks, N.K. Crystal structure of human protein tyrosine phosphatase 1B. Science 1994, 263, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.H.; Ha, M.T.; Min, B.S.; Jung, H.A.; Choi, J.S. Moracin derivatives from Morus Radix as dual BACE1 and cholinesterase inhibitors with antioxidant and anti-glycation capacities. Life Sci. 2018, 210, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, C.G.M.; Haenen, G.R.M.M.; Vekemans, J.A.J.M.; Bast, A. Peroxynitrite scavenging of flavonoids: Structure activity relationship. Environ. Toxicol. Pharmacol. 2001, 10, 199–206. [Google Scholar] [CrossRef]
- Choi, J.S.; Chung, H.Y.; Kang, S.S.; Jung, M.J.; Kim, J.W.; No, J.K.; Jung, H.A. The structure–activity relationship of flavonoids as scavengers of peroxynitrite. Phytother. Res. 2002, 16, 232–235. [Google Scholar] [CrossRef]
- Stevens, J.F.; Miranda, C.L.; Frei, B.; Buhler, D.R. Inhibition of peroxynitrite-mediated LDL oxidation by prenylated flavonoids: The α, β-unsaturated keto functionality of 2 ‘-hydroxychalcones as a novel antioxidant pharmacophore. Chem. Res. Toxicol. 2003, 16, 1277–1286. [Google Scholar] [CrossRef]





| Fractions | IC50 Values (μg/mL) 1 | |||
|---|---|---|---|---|
| α-Glucosidase | PTP1B | AGEs | ONOO− | |
| MeOH ext. | 100.3 ± 0.55 | 55.29 ± 27.46 | 23.06 ± 0.84 | 12.01 ± 1.43 |
| CH2Cl2 fr. | 135.3 ± 0.69 | 12.86 ± 2.45 | 48.64 ± 6.38 | 14.63 ± 0.92 |
| EtOAc fr. | 2.74 ± 0.15 | 8.09 ± 0.08 | 6.40 ± 0.31 | 6.74 ± 0.15 |
| n-BuOH fr. | 241.7 ± 0.10 | 15.05 ± 1.57 | 36.65 ± 2.00 | 10.98 ± 0.46 |
| H2O fr. | 457.9 ± 0.98 | >100 | >100 | 22.11 ± 3.56 |
| Acarbose 2 | 494.1 ± 1.19 | - | - | - |
| Ursolic acid 2 | - | 4.56 ± 0.22 | - | - |
| Aminoguanidine 2 | - | - | 52.62 ± 6.70 | - |
| l-Penicillamine 2 | - | - | - | 1.37 ± 0.19 |
| Compounds | Binding Energy 1 | Number of H-Bonds | H-Bond Interacting Residues | Hydrophobic Interacting Residues | Electrostatic Interacting Residues |
|---|---|---|---|---|---|
| Kuwanon C (1) | −6.66 | 4 | Gln279, Arg315, Arg442, Asp307 | Tyr158 (Pi-Pi T-shaped, Pi-Alkyl), Lys156 (Alkyl), Phe303 (Pi-Alkyl), Arg315 (Pi-Alkyl) | |
| −8.47 | 5 | Glu296, Ser298, Leu297, Glu271, Arg270 | Ala292 (Alkyl), Lys13 (Alkyl), Ile263 (Alkyl), Ile272 (Alkyl), Ile262 (Alkyl), Arg263 (Alkyl) | Glu271 (Pi-Anion) | |
| Moracin M (2) | −7.73 | 5 | Arg442, Asp69, Gln182, Asp215, Glu411 | Tyr72 (Pi-Pi T-shaped), Tyr158 (Pi-Pi T-shaped) | Arg442 (Pi-Cation) |
| −7.48 | 4 | Lys16, His295, Asn259, Thr274 | Trp15 (Pi-Sigma, Pi-Pi T-shaped), Ala292 (Pi-Sigma) | ||
| Dihydromorin (3) | −6.52 | 6 | Gln279, Arg315, Arg442, Asp69, Glu277, Asp352 | Tyr72 (Pi-Pi T-shaped) | Arg442 (Pi-Cation), Asp352 (Pi-Anion), Glu411 (Pi-Anion) |
| −6.93 | 4 | Glu296, Asn259, Glu271, Ser291 | Ala292 (Pi-Sigma), Arg263 (Pi-Alkyl) | ||
| Oxyresveratrol (5) | −7.72 | 4 | Asp352, Asp215, Gln353, Glu411 | Tyr72 (Pi-Pi T-shaped), Phe178 (Pi-Pi T-shaped), Val216 (Pi-Alkyl), Arg442 (Pi-Alkyl) | Arg442 (Pi-Cation), Asp69 (Pi-Anion), Glu277 (Pi-Anion), Asp352 (Pi-Anion) |
| −6.98 | 6 | Thr274, Thr290, Cys342, Ile272, Asn259, Glu296 | Ala292 (Pi-Sigma), Trp15 (Pi-Pi T-shaped), Ser291 (Amide-Pi Stacked) | ||
| Norartocarpetin (6) | −6.64 | 1 | Gln353 | Val216 (PI-Alkyl) | Arg442 (Pi-Cation), Glu277 (Pi-Anion), Asp352 (Pi-Anion) |
| −7.39 | 4 | Lys13, Lys16, Thr274, Glu11 | Ala292 (Pi-Sigma), Lys13 (Pi-Alkyl) | Glu271 (Pi-Anion) | |
| Kuwanon G (7) | −5.99 | 6 | Asn350, Gln353, Glu277, Asp352, Asp242, Glu411 | Phe303 (Pi-Pi Stacked) | |
| −8.89 | 5 | Ser298, Asn259, Ile272, Asp341, Thr290, Ala292, Arg270 | Ala292 (Pi-sigma, Pi-Alkyl, Alkyl), Trp15 (Pi-Pi T-shaped), His295 (Pi-Alkyl), Ile262 (Pi-Alkyl), Ile272 (Pi-Alkyl), Arg263 (Pi-Alkyl) | Lys13 (Pi-Cation), Glu271 (Pi-Anion) | |
| Acarbose 2 | −8.6 | 6 | His112, Ser241, Arg442, Asp352, Asp242, Asp69 | Tyr158 (Pi-Sigma), Phe303 (Pi-Alkyl) | |
| BIP 3 | −6.03 | 6 | Lys16 | Ala292 (Pi-Sigma, Alkyl), Trp15 (Pi-Pi T-shaped, Pi-Alkyl), Lys13 (Alkyl) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, R.-H.; Thaku, N.; Timalsina, B.; Park, S.-E.; Choi, J.-S.; Jung, H.-A. Inhibition Mechanism of Components Isolated from Morus alba Branches on Diabetes and Diabetic Complications via Experimental and Molecular Docking Analyses. Antioxidants 2022, 11, 383. https://doi.org/10.3390/antiox11020383
Kwon R-H, Thaku N, Timalsina B, Park S-E, Choi J-S, Jung H-A. Inhibition Mechanism of Components Isolated from Morus alba Branches on Diabetes and Diabetic Complications via Experimental and Molecular Docking Analyses. Antioxidants. 2022; 11(2):383. https://doi.org/10.3390/antiox11020383
Chicago/Turabian StyleKwon, Ryeong-Ha, Niha Thaku, Binod Timalsina, Se-Eun Park, Jae-Sue Choi, and Hyun-Ah Jung. 2022. "Inhibition Mechanism of Components Isolated from Morus alba Branches on Diabetes and Diabetic Complications via Experimental and Molecular Docking Analyses" Antioxidants 11, no. 2: 383. https://doi.org/10.3390/antiox11020383
APA StyleKwon, R. -H., Thaku, N., Timalsina, B., Park, S. -E., Choi, J. -S., & Jung, H. -A. (2022). Inhibition Mechanism of Components Isolated from Morus alba Branches on Diabetes and Diabetic Complications via Experimental and Molecular Docking Analyses. Antioxidants, 11(2), 383. https://doi.org/10.3390/antiox11020383