The Protective Effect of Anethole against Renal Ischemia/Reperfusion: The Role of the TLR2,4/MYD88/NFκB Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ethical Approval Declaration
2.3. Induction Renal Ischemia/Reperfusion (RIR) Injury
2.4. Experimental Design
2.5. Evaluations Using Histopathological and Immunohistochemical Investigations
2.6. Evaluation of Renal Function
2.7. Evaluation of Renal Oxidative Stress Status
2.8. Evaluation of Toll-like Receptors (TLR) Pathway Gene Expression
2.9. Evaluation of Toll-like Receptors (TLR) Pathway Protein Expression
2.10. In Silico Evaluation of Toll-like Receptors (TLR) Interaction with Anethole
2.11. Evaluation of Inflammatory Indicators
2.12. Evaluation of Apoptotic Indicators
2.13. Statistical Analysis
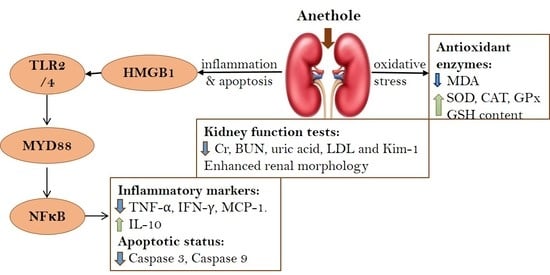
3. Results
3.1. Anethole Averts RIR-Induced Renal Histological Alterations

3.2. Anethole Averts RIR-Induced Renal Function Alterations
3.3. Anethole Improves RIR Induced Renal Oxidative Stress
3.4. Anethole Averts RIR Induced Renal Toll like Receptors (TLR) Alterations
3.5. In Silico Anethole Interaction with TLR
3.6. Anethole Averts RIR Induced Renal Inflammation
3.7. Anethole Averts RIR Induced Renal Function Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gholampour, H.; Moezi, L.; Shafaroodi, H. Aripiprazole prevents renal ischemia/reperfusion injury in rats, probably through nitric oxide involvement. Eur. J. Pharmacol. 2017, 813, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Kolankaya, D. The protective effects of ascorbic acid against renal ischemia-reperfusion injury in male rats. Ren. Fail. 2009, 31, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Baud, L.; Ardaillou, R. Involvement of reactive oxygen species in kidney damage. Br. Med. Bull. 1993, 49, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Shen, X.D.; O’Connell, R.; Gao, F.; Lassman, C.; Busuttil, R.W.; Cheng, G.; Kupiec-Weglinski, J.W. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J. Immunol. 2004, 173, 7115–7119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, C.; Yang, J.; Yang, H.; Li, X.; Wang, G. Attenuation of renal ischemia/reperfusion injury by oleanolic acid preconditioning via its antioxidant, anti-inflammatory, and anti-apoptotic activities. Mol. Med. Rep. 2016, 13, 4697–4704. [Google Scholar] [CrossRef]
- Mishra, J.; Mori, K.; Ma, Q.; Kelly, C.; Yang, J.; Mitsnefes, M.; Barasch, J.; Devarajan, P. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J. Am. Soc. Nephrol. JASN 2004, 15, 3073–3082. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.J.; Koo, S.; Cha, H.J.; Lee, J.S.; Kwon, B.; Cho, H.R. Inhibition of kidney ischemia–reperfusion injury through local infusion of a TLR2 blocker. J. Immunol. Methods 2014, 407, 146–150. [Google Scholar] [CrossRef]
- Wu, H.; Chen, G.; Wyburn, K.R.; Yin, J.; Bertolino, P.; Eris, J.M.; Alexander, S.I.; Sharland, A.F.; Chadban, S.J. TLR4 activation mediates kidney ischemia/reperfusion injury. J. Clin. Investig. 2007, 117, 2847–2859. [Google Scholar] [CrossRef]
- Wu, H.; Ma, J.; Wang, P.; Corpuz, T.M.; Panchapakesan, U.; Wyburn, K.R.; Chadban, S.J. HMGB1 contributes to kidney ischemia reperfusion injury. J. Am. Soc. Nephrol. JASN 2010, 21, 1878–1890. [Google Scholar] [CrossRef] [Green Version]
- Chao, W. Toll-like receptor signaling: A critical modulator of cell survival and ischemic injury in the heart. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1–H12. [Google Scholar] [CrossRef] [Green Version]
- Marsh, B.J.; Stevens, S.L.; Hunter, B.; Stenzel-Poore, M.P. Inflammation and the emerging role of the toll-like receptor system in acute brain ischemia. Stroke 2009, 40, S34–S37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusai, K.; Sollinger, D.; Baumann, M.; Wagner, B.; Strobl, M.; Schmaderer, C.; Roos, M.; Kirschning, C.; Heemann, U.; Lutz, J. Toll-like receptors 2 and 4 in renal ischemia/reperfusion injury. Pediatric Nephrol. 2010, 25, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, H.; Fadillioglu, E.; Yagmurca, M.; Uçar, M.; Irmak, M.K. Protein oxidation and lipid peroxidation after renal ischemia-reperfusion injury: Protective effects of erdosteine and N-acetylcysteine. Urol. Res. 2006, 34, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski-Rebecca, E.S.; Rocha, B.A.; Wiirzler, L.A.; Cuman, R.K.; Velazquez-Martinez, C.A.; Bersani-Amado, C.A. Synergistic effects of anethole and ibuprofen in acute inflammatory response. Chem.-Biol. Interact. 2015, 242, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.M.V.; Domiciano, T.P.; Verri, W.A.; Zarpelon, A.C.; da Silva, L.G.; Barbosa, C.P.; Natali, M.R.M.; Cuman, R.K.N.; Bersani-Amado, C.A. Antihypernociceptive activity of anethole in experimental inflammatory pain. Inflammopharmacology 2013, 21, 187–197. [Google Scholar] [CrossRef]
- Choo, E.J.; Rhee, Y.-H.; Jeong, S.-J.; Lee, H.-J.; Kim, H.S.; Ko, H.S.; Kim, J.-H.; Kwon, T.-R.; Jung, J.H.; Kim, J.H.; et al. Anethole Exerts Antimetatstaic Activity via Inhibition of Matrix Metalloproteinase 2/9 and AKT/Mitogen-Activated Kinase/Nuclear Factor Kappa B Signaling Pathways. Biol. Pharm. Bull. 2011, 34, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Freire, R.S.; Morais, S.M.; Catunda-Junior, F.E.A.; Pinheiro, D.C.S.N. Synthesis and antioxidant, anti-inflammatory and gastroprotector activities of anethole and related compounds. Bioorg. Med. Chem. 2005, 13, 4353–4358. [Google Scholar] [CrossRef]
- Kosalec, I.; Pepeljnjak, S.; Kustrak, D. Antifungal activity of fluid extract and essential oil from anise fruits (Pimpinella anisum L., Apiaceae). Acta Pharm. 2005, 55, 377–385. [Google Scholar]
- Astani, A.; Reichling, J.; Schnitzler, P. Screening for Antiviral Activities of Isolated Compounds from Essential Oils. Evid.-Based Complement. Altern. Med. 2011, 2011, 253643. [Google Scholar] [CrossRef] [Green Version]
- Ghelardini, C.; Galeotti, N.; Mazzanti, G. Local anaesthetic activity of monoterpenes and phenylpropanes of essential oils. Planta Med. 2001, 67, 564–566. [Google Scholar] [CrossRef]
- Seo, E.; Kang, P.; Seol, G.H. Trans-anethole prevents hypertension induced by chronic exposure to both restraint stress and nicotine in rats. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 102, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.I.; Kim, K.M.; Kwak, J.H.; Lee, S.K.; Lee, S.M. Protective mechanism of anethole on hepatic ischemia/reperfusion injury in mice. J. Nat. Prod. 2013, 76, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Seol, G.H.; Park, H.; Choi, I.-Y. Trans-anethole protects cortical neuronal cells against oxygen–glucose deprivation/reoxygenation. Neurol. Sci. 2014, 35, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.M.V.; Hernandes, L.; da Rocha, B.A.; Estevão-Silva, C.F.; Wisniewski-Rebecca, E.S.; Cezar, J.D.S.; Caparroz-Assef, S.M.; Cuman, R.K.N.; Bersani-Amado, C.A. Anethole reduces inflammation and joint damage in rats with adjuvant-induced arthritis. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2017, 66, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yang, C.; Zhao, T.; Xu, M.; Tang, Q.; Yang, B.; Rong, R.; Zhu, T. Erythropoietin Ameliorates Renal Ischemia and Reperfusion Injury via Inhibiting Tubulointerstitial Inflammation. J. Surg. Res. 2012, 176, 260–266. [Google Scholar] [CrossRef]
- da Rocha, B.A.; Ritter, A.M.; Ames, F.Q.; Gonçalves, O.H.; Leimann, F.V.; Bracht, L.; Natali, M.R.; Cuman, R.K.; Bersani-Amado, C.A. Acetaminophen-induced hepatotoxicity: Preventive effect of trans anethole. Biomed. Pharmacother. 2017, 86, 213–220. [Google Scholar] [CrossRef]
- Estevão-Silva, C.F.; Kummer, R.; Fachini-Queiroz, F.C.; Grespan, R.; Nogueira de Melo, G.A.; Baroni, S.; Cuman, R.K.; Bersani-Amado, C.A. Anethole and eugenol reduce in vitro and in vivo leukocyte migration induced by fMLP, LTB4, and carrageenan. J. Nat. Med. 2014, 68, 567–575. [Google Scholar] [CrossRef]
- Tan, Z.; Shi, Y.; Yan, Y.; Liu, W.; Li, G.; Li, R. Impact of endogenous hydrogen sulfide on toll-like receptor pathway in renal ischemia/reperfusion injury in rats. Ren. Fail. 2015, 37, 727–733. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.E.; Abduldaium, Y.S.; Younis, N.S. Ameliorative Effect of Linalool in Cisplatin-Induced Nephrotoxicity: The Role of HMGB1/TLR4/NF-κB and Nrf2/HO1 Pathways. Biomolecules 2020, 10, 1488. [Google Scholar] [CrossRef]
- Younis, N.S.; Mohamed, M.E. β-Caryophyllene as a Potential Protective Agent against Myocardial Injury: The Role of Toll-Like Receptors. Molecules 2019, 24, 1929. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.; Zhu, H.; Tao, Q.; Guo, L.; Jiang, T.; Xu, L.; Yang, R.; Wei, X.; Wu, J.; Li, X.; et al. FGF10 Protects Against Renal Ischemia/Reperfusion Injury by Regulating Autophagy and Inflammatory Signaling. Front. Genet. 2018, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Li, L.; Li, L.; Pokhrel, G.; Qi, G.; Rong, R.; Zhu, T. The protective effect of baicalin against renal ischemia-reperfusion injury through inhibition of inflammation and apoptosis. BMC Complement. Altern. Med. 2014, 14, 19. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, B.A.; Pari, L.; Rathinam, A.; Chandramohan, R. Trans-anethole, a terpenoid ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin induced diabetic rats. Biochimie 2015, 112, 57–65. [Google Scholar] [CrossRef]
- Samadi-Noshahr, Z.; Ebrahimzadeh-Bideskan, A.; Hadjzadeh, M.A.; Shafei, M.N.; Salmani, H.; Hosseinian, S.; Khajavi-Rad, A. trans-Anethole attenuated renal injury and reduced expressions of angiotensin II receptor (AT1R) and TGF-β in streptozotocin-induced diabetic rats. Biochimie 2021, 185, 117–127. [Google Scholar] [CrossRef]
- Chainy, G.B.; Manna, S.K.; Chaturvedi, M.M.; Aggarwal, B.B. Anethole blocks both early and late cellular responses transduced by tumor necrosis factor: Effect on NF-kappaB, AP-1, JNK, MAPKK and apoptosis. Oncogene 2000, 19, 2943–2950. [Google Scholar] [CrossRef] [Green Version]







| Animal Experimental Groups | 1% CMC in Saline Orally (for 14 Days before Surgery) | Anethole Orally # (for 14 Days before Surgery) | Renal Arteries Clamping (for 45 Min, then Reperfusion) | |
|---|---|---|---|---|
| 125 mg/kg | 250 mg/kg | |||
| Sham | Yes | No | No | No |
| Sham + anethole | No | No | Yes | No |
| RIR | Yes | No | No | Yes |
| RIR + anethole (125 mg/kg) | No | Yes | No | Yes |
| RIR + anethole (250 mg/kg) | No | No | Yes | Yes |
| TLR Pathway | Primer Sequence (5′ to 3′) | Gen-Bank Accession Number |
|---|---|---|
| HMGB-1 | 5′-AGGCTGACAAGGCTCGTTATG-3′ (sense) 5′-TGTCATCCGCAGCAGTGTTG-3′ (antisense) | XM_039100270 |
| TLR2 | 5′-ATGAACACTAAGACATACCTGGAG-3′ (sense) 5′-CAAGACAGAAACAGGGTGGAG-3′ (antisense) | NM_198769 |
| TLR4 | 5′-CATGACATCCCTTATTCAACCAAG-3′ (sense), 5′-GCCATGCCTTGTCTTCAATTG-3′ (antisense) | NM_019178 |
| MyD88 | 5′-GAGATCCGCGAGTTTGAGAC-3′ (sense) 5′-CTGTTTCTGCTGGTTGCGTA-3′ (antisense) | NM_198130.2 |
| NFκB | 5′-ATCATCAACATGAGAAACGATCTGTA-3′ (sense) 5′-CAGCGGTCCAGAAGACTCAG-3′ (antisense) | L26267.1 |
| β-Actin | 5′-TGCTATGTT GCCCTAGACTTCG-3′ (sense) 5′-GTTGGCATAGAG GTCTTTACGG-3′ (antisense) | NM_031144 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, M.E.; Kandeel, M.; Abd El-Lateef, H.M.; El-Beltagi, H.S.; Younis, N.S. The Protective Effect of Anethole against Renal Ischemia/Reperfusion: The Role of the TLR2,4/MYD88/NFκB Pathway. Antioxidants 2022, 11, 535. https://doi.org/10.3390/antiox11030535
Mohamed ME, Kandeel M, Abd El-Lateef HM, El-Beltagi HS, Younis NS. The Protective Effect of Anethole against Renal Ischemia/Reperfusion: The Role of the TLR2,4/MYD88/NFκB Pathway. Antioxidants. 2022; 11(3):535. https://doi.org/10.3390/antiox11030535
Chicago/Turabian StyleMohamed, Maged Elsayed, Mahmoud Kandeel, Hany M. Abd El-Lateef, Hossam S. El-Beltagi, and Nancy S. Younis. 2022. "The Protective Effect of Anethole against Renal Ischemia/Reperfusion: The Role of the TLR2,4/MYD88/NFκB Pathway" Antioxidants 11, no. 3: 535. https://doi.org/10.3390/antiox11030535
APA StyleMohamed, M. E., Kandeel, M., Abd El-Lateef, H. M., El-Beltagi, H. S., & Younis, N. S. (2022). The Protective Effect of Anethole against Renal Ischemia/Reperfusion: The Role of the TLR2,4/MYD88/NFκB Pathway. Antioxidants, 11(3), 535. https://doi.org/10.3390/antiox11030535