Exogenous Melatonin Ameliorates the Negative Effect of Osmotic Stress in Human and Bovine Ovarian Stromal Cells
Abstract
:1. Introduction
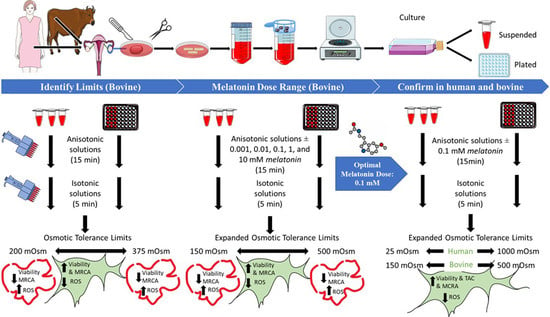
2. Materials and Methods
2.1. Chemicals
2.2. Ovarian Tissue Collection
2.2.1. Bovine
2.2.2. Human
2.3. Ovarian Tissue Processing and Stromal Cell Isolation Procedure
2.4. Ovarian Stromal Cell Viability and Concentration after Trypsinization
2.5. Anisotonic Solutions Preparation
2.6. Phase 1: Detection of OTLs of Bovine OSCs, Together with the ROS Production Levels and MRCA Activity after Exposure to the Different Range of Anisotonic Solutions
2.6.1. Osmotic Sensitivity Assessment
2.6.2. Reactive Oxygen Species Levels Measurement
2.6.3. Mitochondrial Respiratory Chain Activity Measurement
2.7. Phase 2
Identification of Optimum Concentration of Melatonin
2.8. Phase 3
Total Antioxidant Capacity Measurement
2.9. Statistical Analysis
3. Results
3.1. Osmotic Tolerance Limits (Phase 1)
3.2. Oxidative Stress Measurement
3.3. Mitochondrial Respiratory Chain Activity
3.4. Optimal Concentration of Melatonin (Phase 2)
3.5. Phase 3
3.5.1. Effect of Optimal Dose of Melatonin on Ovarian Stromal Cells Osmotic Tolerance Limits (Cell Viability)
3.5.2. Effect of Optimal Dose of Melatonin on Osmotic Stress-Induced ROS
3.5.3. Effect of Optimal Dose of Melatonin on Mitochondrial Respiratory Chain Activity
3.5.4. Effect of Optimal Dose of Melatonin on Total Antioxidant Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.; Ryu, K.-J.; Kim, B.; Kang, D.; Kim, Y.Y.; Kim, T. Comparison between slow freezing and vitrification for human ovarian tissue cryopreservation and xenotransplantation. Int. J. Mol. Sci. 2019, 20, 3346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoekman, E.J.; Louwe, L.A.; Rooijers, M.; van der Westerlaken, L.A.; Klijn, N.F.; Pilgram, G.S.; de Kroon, C.D.; Hilders, C.G. Ovarian tissue cryopreservation: Low usage rates and high live-birth rate after transplantation. Acta Obstet. Gynecol. Scand. 2020, 99, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Kelvin, J.F.; Quinn, G.P.; Gracia, C.R. Infertility in reproductive-age female cancer survivors. Cancer 2015, 121, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.; Shalet, S. Gonadal damage from chemotherapy and radiotherapy. Endocrinol. Metab. Clin. N. Am. 1998, 27, 927–943. [Google Scholar] [CrossRef]
- Suh, E.; Stratton, K.L.; Leisenring, W.M.; Nathan, P.C.; Ford, J.S.; Freyer, D.R.; McNeer, J.L.; Stock, W.; Stovall, M.; Krull, K.R. Late mortality and chronic health conditions in long-term survivors of early-adolescent and young adult cancers: A retrospective cohort analysis from the Childhood Cancer Survivor Study. Lancet Oncol. 2020, 21, 421–435. [Google Scholar] [CrossRef]
- Salama, M.; Woodruff, T.K. Anticancer treatments and female fertility: Clinical concerns and role of oncologists in oncofertility practice. Expert Rev. Anticancer Ther. 2017, 17, 687–692. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Lee, S.; Kim, T. Ovarian tissue cryopreservation and transplantation in patients with cancer. Obstet. Gynecol. Sci. 2018, 61, 431–442. [Google Scholar] [CrossRef]
- Dolmans, M.-M.; Donnez, J.; Cacciottola, L. Fertility preservation: The challenge of freezing and transplanting ovarian tissue. Trends Mol. Med. 2021, 27, 777–791. [Google Scholar] [CrossRef]
- Labrune, E.; Jaeger, P.; Santamaria, C.; Fournier, C.; Benchaib, M.; Rabilloud, M.; Salle, B.; Lornage, J. Cellular and molecular impact of vitrification versus slow freezing on ovarian tissue. Tissue Eng. Part C Methods 2020, 26, 276–285. [Google Scholar] [CrossRef]
- Gavish, Z.; Spector, I.; Peer, G.; Schlatt, S.; Wistuba, J.; Roness, H.; Meirow, D. Follicle activation is a significant and immediate cause of follicle loss after ovarian tissue transplantation. J. Assist. Reprod. Genet. 2018, 35, 61–69. [Google Scholar] [CrossRef]
- Karlsson, J.O.; Toner, M. Long-term storage of tissues by cryopreservation: Critical issues. Biomaterials 1996, 17, 243–256. [Google Scholar] [CrossRef]
- Pegg, D.E. Principles of cryopreservation. In Cryopreservation and Freeze-Drying Protocols; Springer: Amsterdam, The Netherlands, 2015; pp. 3–19. [Google Scholar]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and its clinical applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, S.; Diaz-Garcia, C.; Pellicer, A. Ovarian tissue cryopreservation: Slow freezing. In Gonadal Tissue Cryopreservation in Fertility Preservation; Springer: Amsterdam, The Netherlands, 2016; pp. 53–77. [Google Scholar]
- Arav, A.; Natan, Y. Directional freezing: A solution to the methodological challenges to preserve large organs. In Seminars in Reproductive Medicine; Thieme Medical Publishers: New York, NY, USA, 2009; pp. 438–442. [Google Scholar]
- Medicine, P.C.o.t.A.S.f.R. Ovarian tissue cryopreservation: A committee opinion. Fertil. Steril. 2014, 101, 1237–1243. [Google Scholar]
- Benson, J.D.; Higgins, A.Z.; Desai, K.; Eroglu, A. A toxicity cost function approach to optimal CPA equilibration in tissues. Cryobiology 2018, 80, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Kashuba, C.M.; Benson, J.D.; Critser, J.K. Rationally optimized cryopreservation of multiple mouse embryonic stem cell lines: I—Comparative fundamental cryobiology of multiple mouse embryonic stem cell lines and the implications for embryonic stem cell cryopreservation protocols. Cryobiology 2014, 68, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahmann, J.M.; Benson, J.D.; Higgins, A.Z. Concentration dependence of the cell membrane permeability to cryoprotectant and water and implications for design of methods for post-thaw washing of human erythrocytes. Cryobiology 2018, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.F.; Benson, J.D.; Higgins, A.Z. Mathematically optimized cryoprotectant equilibration procedures for cryopreservation of human oocytes. Theor. Biol. Med. Model. 2014, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- Gilmore, J.; Liu, J.; Peter, A.; Critser, J. Determination of plasma membrane characteristics of boar spermatozoa and their relevance to cryopreservation. Biol. Reprod. 1998, 58, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Bissoyi, A.; Nayak, B.; Pramanik, K.; Sarangi, S.K. Targeting cryopreservation-induced cell death: A review. Biopreservation Biobanking 2014, 12, 23–34. [Google Scholar] [CrossRef]
- Baust, J.G.; Gao, D.; Baust, J.M. Cryopreservation: An emerging paradigm change. Organogenesis 2009, 5, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Zarif-Yeganeh, M.; Rastegarpanah, M. Clinical role of silymarin in oxidative stress and infertility: A short review for pharmacy practitioners. J. Res. Pharm. Pract. 2019, 8, 181. [Google Scholar] [PubMed]
- Gupta, S.; Ghulmiyyah, J.; Sharma, R.; Halabi, J.; Agarwal, A. Power of proteomics in linking oxidative stress and female infertility. BioMed Res. Int. 2014, 2014, 916212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lettieri, G.; D’Agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T. Discovery of the involvement in DNA oxidative damage of human sperm nuclear basic proteins of healthy young men living in polluted areas. Int. J. Mol. Sci. 2020, 21, 4198. [Google Scholar] [CrossRef]
- Lettieri, G.; Marra, F.; Moriello, C.; Prisco, M.; Notari, T.; Trifuoggi, M.; Giarra, A.; Bosco, L.; Montano, L.; Piscopo, M. Molecular Alterations in Spermatozoa of a Family Case Living in the Land of Fires—A First Look at Possible Transgenerational Effects of Pollutants. Int. J. Mol. Sci. 2020, 21, 6710. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.C.; Liu, X.C.; Yang, S.H.; Song, L.L.; Zhou, S.J.; Deng, S.L.; Tian, L.; Cheng, L.Y. Melatonin inhibits oxidative stress and apoptosis in cryopreserved ovarian tissues via Nrf2/HO-1 signaling pathway. Front. Mol. Biosci. 2020, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.C.; Sun, T.C.; Li, H.Y.; Si, L.N.; Wei, M.; Chen, Z.H.; Cheng, L.Y.; Yang, S.H. Antioxidative effect of melatonin on cryopreserved ovarian tissue in mice. Cryobiology 2020, 96, 99–105. [Google Scholar] [CrossRef]
- Asadi, E.; Najafi, A.; Moeini, A.; Pirjani, R.; Hassanzadeh, G.; Mikaeili, S.; Salehi, E.; Adutwum, E.; Soleimani, M.; Khosravi, F. Ovarian tissue culture in the presence of VEGF and fetuin stimulates follicle growth and steroidogenesis. J. Endocrinol. 2017, 232, 205–219. [Google Scholar] [CrossRef] [Green Version]
- Thomson, L.K.; Fleming, S.D.; Aitken, R.J.; De Iuliis, G.N.; Zieschang, J.-A.; Clark, A.M. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum. Reprod. 2009, 24, 2061–2070. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Sharma, R.K.; Sikka, S.C.; Thomas, A.J., Jr.; Falcone, T.; Agarwal, A. Oxidative stress is associated with increased apoptosis leading to spermatozoa DNA damage in patients with male factor infertility. Fertil. Steril. 2003, 80, 531–535. [Google Scholar] [CrossRef]
- Gavish, Z.; Peer, G.; Hadassa, R.; Yoram, C.; Meirow, D. Follicle activation and ‘burn-out’contribute to post-transplantation follicle loss in ovarian tissue grafts: The effect of graft thickness. Hum. Reprod. 2014, 29, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Tomás, C.; Blanch, E.; Hernández, M.; Gil, M.A.; Roca, J.; Vázquez, J.M.; Martínez, E.A.; Mocé, E. Treating boar sperm with cholesterol-loaded cyclodextrins widens the sperm osmotic tolerance limits and enhances the in vitro sperm fertilising ability. Anim. Reprod. Sci. 2011, 129, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeel, E.; Schneider, U.; Liu, J.; Agca, Y.; Critser, J.K.; Van Steirteghem, A. Osmotic responses and tolerance limits to changes in external osmolalities, and oolemma permeability characteristics, of human in vitro matured MII oocytes. Hum. Reprod. 2007, 22, 1959–1972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Men, H.; Agca, Y.; Mullen, S.F.; Critser, E.S.; Critser, J.K. Osmotic tolerance of in vitro produced porcine blastocysts assessed by their morphological integrity and cellular actin filament organization. Cryobiology 2005, 51, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Benson, J.D.; Men, H.; Critser, J.K. Osmotic tolerance limits and effects of cryoprotectants on the motility, plasma membrane integrity and acrosomal integrity of rat sperm. Cryobiology 2006, 53, 336–348. [Google Scholar] [CrossRef]
- Zhurova, M.; Lusianti, R.E.; Higgins, A.Z.; Acker, J.P. Osmotic tolerance limits of red blood cells from umbilical cord blood. Cryobiology 2014, 69, 48–54. [Google Scholar] [CrossRef]
- Burnaugh, L.; Ball, B.; Sabeur, K.; Thomas, A.; Meyers, S.A. Osmotic stress stimulates generation of superoxide anion by spermatozoa in horses. Anim. Reprod. Sci. 2010, 117, 249–260. [Google Scholar] [CrossRef]
- McCarthy, M.J.; Baumber, J.; Kass, P.H.; Meyers, S.A. Osmotic stress induces oxidative cell damage to rhesus macaque spermatozoa. Biol. Reprod. 2010, 82, 644–651. [Google Scholar] [CrossRef] [Green Version]
- Len, J.S.; Koh, W.S.D.; Tan, S.-X. The roles of reactive oxygen species and antioxidants in cryopreservation. Biosci. Rep. 2019, 39, BSR20191601. [Google Scholar] [CrossRef] [Green Version]
- Lambert, I.; Pedersen, S.; Poulsen, K. Activation of PLA2 isoforms by cell swelling and ischaemia/hypoxia. Acta Physiol. 2006, 187, 75–85. [Google Scholar] [CrossRef]
- Leonel, E.C.R.; Lucci, C.M.; Amorim, C.A. Cryopreservation of human ovarian tissue: A review. Transfus. Med. Hemother. 2019, 46, 173–181. [Google Scholar]
- Meyers, S.A. Spermatozoal response to osmotic stress. Anim. Reprod. Sci. 2005, 89, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Yoshimori, T.; Takamatsu, H. 3-D measurement of osmotic dehydration of isolated and adhered PC-3 cells. Cryobiology 2009, 58, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Hayashi, H.; Yoshida, T. The effect of osmolarity on metabolism and morphology in adhesion and suspension chinese hamster ovary cells producing tissue plasminogen activator. Cytotechnology 2000, 32, 171–179. [Google Scholar] [CrossRef]
- Marsella, T.; Sena, P.; Xella, S.; La Marca, A.; Giulini, S.; De Pol, A.; Volpe, A.; Marzona, L. Human ovarian tissue cryopreservation: Effect of sucrose concentration on morphological features after thawing. Reprod. Biomed. Online 2008, 16, 257–267. [Google Scholar] [CrossRef]
- Shahri, P.A.K.; Chiti, M.C.; Amorim, C.A. Isolation and characterization of the human ovarian cell population for transplantation into an artificial ovary. Anim. Reprod. 2019, 16, 39. [Google Scholar] [CrossRef]
- Wagner, M.; Yoshihara, M.; Douagi, I.; Damdimopoulos, A.; Panula, S.; Petropoulos, S.; Lu, H.; Pettersson, K.; Palm, K.; Katayama, S. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat. Commun. 2020, 11, 1147. [Google Scholar] [CrossRef] [Green Version]
- Knight, P.G.; Glister, C. TGF-β superfamily members and ovarian follicle development. Reproduction 2006, 132, 191–206. [Google Scholar] [CrossRef] [Green Version]
- Orisaka, M.; Tajima, K.; Mizutani, T.; Miyamoto, K.; Tsang, B.K.; Fukuda, S.; Yoshida, Y.; Kotsuji, F. Granulosa cells promote differentiation of cortical stromal cells into theca cells in the bovine ovary. Biol. Reprod. 2006, 75, 734–740. [Google Scholar] [CrossRef] [Green Version]
- Tagler, D.J.; Shea, L.D.; Woodruff, T.K. Contributions of ovarian stromal cells to follicle culture. In Principles and Practice of Fertility Preservation; Cambridge University Press: Cambridge, MA, USA, 2011; pp. 409–420. [Google Scholar]
- Kinnear, H.M.; Tomaszewski, C.E.; Chang, F.L.; Moravek, M.B.; Xu, M.; Padmanabhan, V.; Shikanov, A. The ovarian stroma as a new frontier. Reproduction 2020, 160, R25–R39. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, J.; Han, C.; Wu, B.; Yang, Z.; Su, F.; Quan, F.; Zhang, Y. The influence of ovarian stromal/theca cells during in vitro culture on steroidogenesis, proliferation and apoptosis of granulosa cells derived from the goat ovary. Reprod. Domest. Anim. 2014, 49, 170–176. [Google Scholar] [CrossRef]
- Young, J.; McNeilly, A.S. Theca: The forgotten cell of the ovarian follicle. Reproduction 2010, 140, 489–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keros, V.; Xella, S.; Hultenby, K.; Pettersson, K.; Sheikhi, M.; Volpe, A.; Hreinsson, J.; Hovatta, O. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum. Reprod. 2009, 24, 1670–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbri, R.; Pasquinelli, G.; Bracone, G.; Orrico, C.; Di Tommaso, B.; Venturoli, S. Cryopreservation of human ovarian tissue. Cell Tissue Bank. 2006, 7, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Oskam, I.C.; Asadi, B.A.; Santos, R.R. Histologic and ultrastructural features of cryopreserved ovine ovarian tissue: Deleterious effect of 1, 2-propanediol applying different thawing protocols. Fertil. Steril. 2010, 93, 2764–2766. [Google Scholar] [CrossRef] [PubMed]
- Faustino, L.; Santos, R.; Silva, C.; Pinto, L.; Celestino, J.; Campello, C.; Figueiredo, J.; Rodrigues, A. Goat and sheep ovarian tissue cryopreservation: Effects on the morphology and development of primordial follicles and density of stromal cell. Anim. Reprod. Sci. 2010, 122, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.; Amidi, F.; Sedighi Gilani, M.; Moawad, A.; Asadi, E.; Khanlarkhni, N.; Fallah, P.; Rezaiian, Z.; Sobhani, A. Effect of brain-derived neurotrophic factor on sperm function, oxidative stress and membrane integrity in human. Andrologia 2017, 49, e12601. [Google Scholar] [CrossRef] [PubMed]
- Mazoochi, T.; Khamechian, T.; Ehteram, M.; Kashani, H.H. The effect of melatonin on expression of p53 and ovarian preantral follicle development isolated from vitrified ovary. Comp. Clin. Pathol. 2018, 27, 83–88. [Google Scholar] [CrossRef]
- Nekoonam, S.; Nashtaei, M.S.; Zangi, B.M.; Amidi, F. Effect of Trolox on sperm quality in normozospermia and oligozospermia during cryopreservation. Cryobiology 2016, 72, 106–111. [Google Scholar] [CrossRef]
- Mittal, P.K.; Anand, M.; Madan, A.; Yadav, S.; Kumar, J. Antioxidative capacity of vitamin E, vitamin C and their combination in cryopreserved Bhadavari bull semen. Vet. World 2014, 7, 1127–1131. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Li, R.; Fan, X.; Lv, Y.; Zheng, Y.; Hoque, S.; Wu, D.; Zeng, W. Resveratrol improves Boar sperm quality via 5AMP-activated protein kinase activation during cryopreservation. Oxid. Med. Cell. Longev. 2019, 2019, 5921503. [Google Scholar] [CrossRef] [Green Version]
- Tamura, H.; Nakamura, Y.; Korkmaz, A.; Manchester, L.C.; Tan, D.-X.; Sugino, N.; Reiter, R.J. Melatonin and the ovary: Physiological and pathophysiological implications. Fertil. Steril. 2009, 92, 328–343. [Google Scholar] [CrossRef]
- Itoh, M.T.; Ishizuka, B.; Kuribayashi, Y.; Amemiya, A.; Sumi, Y. Melatonin, its precursors, and synthesizing enzyme activities in the human ovary. Mol. Hum. Reprod. 1999, 5, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Tan, D.-X.; Manchester, L.C.; Paredes, S.D.; Mayo, J.C.; Sainz, R.M. Melatonin and reproduction revisited. Biol. Reprod. 2009, 81, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Tan, D.-x.; Osuna, C.; Gitto, E. Actions of melatonin in the reduction of oxidative stress. J. Biomed. Sci. 2000, 7, 444–458. [Google Scholar] [CrossRef]
- El-Raey, M.; Geshi, M.; Somfai, T.; Kaneda, M.; Hirako, M.; Abdel-Ghaffar, A.E.; Sosa, G.A.; El-Roos, M.E.A.; Nagai, T. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol. Reprod. Dev. 2011, 78, 250–262. [Google Scholar] [CrossRef]
- Shi, J.M.; Tian, X.Z.; Zhou, G.B.; Wang, L.; Gao, C.; Zhu, S.E.; Zeng, S.M.; Tian, J.H.; Liu, G.S. Melatonin exists in porcine follicular fluid and improves in vitro maturation and parthenogenetic development of porcine oocytes. J. Pineal Res. 2009, 47, 318–323. [Google Scholar] [CrossRef]
- Pacchiarotti, A.; Carlomagno, G.; Antonini, G.; Pacchiarotti, A. Effect of myo-inositol and melatonin versus myo-inositol, in a randomized controlled trial, for improving in vitro fertilization of patients with polycystic ovarian syndrome. Gynecol. Endocrinol. 2016, 32, 69–73. [Google Scholar] [CrossRef]
- Tan, D.; Reiter, R.J.; Manchester, L.C.; Yan, M.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Haydari, M.; Maresca, V.; Rigano, D.; Taleei, A.; Shahnejat-Bushehri, A.A.; Hadian, J.; Sorbo, S.; Guida, M.; Manna, C.; Piscopo, M. Salicylic acid and melatonin alleviate the effects of heat stress on essential oil composition and antioxidant enzyme activity in Mentha× piperita and Mentha arvensis L. Antioxidants 2019, 8, 547. [Google Scholar] [CrossRef] [Green Version]
- Soares, M.; Sahrari, K.; Chiti, M.C.; Amorim, C.; Ambroise, J.; Donnez, J.; Dolmans, M.-M. The best source of isolated stromal cells for the artificial ovary: Medulla or cortex, cryopreserved or fresh? Hum. Reprod. 2015, 30, 1589–1598. [Google Scholar] [CrossRef] [Green Version]
- Davidson, A.F.; Glasscock, C.; McClanahan, D.R.; Benson, J.D.; Higgins, A.Z. Toxicity minimized cryoprotectant addition and removal procedures for adherent endothelial cells. PLoS ONE 2015, 10, e0142828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najafi, A.; Asadi, E.; Moawad, A.R.; Mikaeili, S.; Amidi, F.; Adutwum, E.; Safa, M.; Sobhani, A.G. Supplementation of freezing and thawing media with brain-derived neurotrophic factor protects human sperm from freeze-thaw-induced damage. Fertil. Steril. 2016, 106, 1658–1665.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Amero, K.K.; Bosley, T.M. Detection of mitochondrial respiratory dysfunction in circulating lymphocytes using resazurin. Arch. Pathol. Lab. Med. 2005, 129, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.Y.; Sun, T.C.; Liu, X.C.; Yu, H.; Zhou, S.J.; Tian, L.; Yang, S.H.; Liu, B.X. Melatonin induction of HSP90 expression exerts cryoprotective effect on ovarian tissue. Cryobiology 2021, 98, 134–138. [Google Scholar] [CrossRef]
- Zhang, J.-M.; Wang, H.-X.; Lu, X.-L. Damage of Granusola and Stroma Cells Exposed to Mouse Ovarian Tissue Cryopreservation: Potential Mechanism of Ovarian Injury. Cryoletters 2021, 42, 53–58. [Google Scholar] [PubMed]
- Xiao, Z.; Wang, Y.; Li, L.; Luo, S.; Li, S.-W. Needle immersed vitrification can lower the concentration of cryoprotectant in human ovarian tissue cryopreservation. Fertil. Steril. 2010, 94, 2323–2328. [Google Scholar] [CrossRef]
- Ragoonanan, V.; Hubel, A.; Aksan, A. Response of the cell membrane–cytoskeleton complex to osmotic and freeze/thaw stresses. Cryobiology 2010, 61, 335–344. [Google Scholar] [CrossRef]
- Finan, J.D.; Guilak, F. The effects of osmotic stress on the structure and function of the cell nucleus. J. Cell. Biochem. 2010, 109, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Cotton, L.M.; Rodriguez, C.M.; Suzuki, K.; Orgebin-Crist, M.C.; Hinton, B.T. Organic cation/carnitine transporter, OCTN2, transcriptional activity is regulated by osmotic stress in epididymal cells. Mol. Reprod. Dev. Inc. Gamete Res. 2010, 77, 114–125. [Google Scholar] [CrossRef]
- Koivusalo, M.; Kapus, A.; Grinstein, S. Sensors, transducers, and effectors that regulate cell size and shape. J. Biol. Chem. 2009, 284, 6595–6599. [Google Scholar] [CrossRef] [Green Version]
- Dmitrieva, N.I.; Michea, L.F.; Rocha, G.M.; Burg, M.B. Cell cycle delay and apoptosis in response to osmotic stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 130, 411–420. [Google Scholar] [CrossRef]
- Han, Y.K.; Kim, Y.G.; Kim, J.Y.; Lee, G.M. Hyperosmotic stress induces autophagy and apoptosis in recombinant Chinese hamster ovary cell culture. Biotechnol. Bioeng. 2010, 105, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Sheikh-Hamad, D.; Gustin, M.C. MAP kinases and the adaptive response to hypertonicity: Functional preservation from yeast to mammals. Am. J. Physiol. Ren. Physiol. 2004, 287, F1102–F1110. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.R.; Bell, J.H.; Frödin, M.; Hansen, S.H. A βPIX-PAK2 complex confers protection against Scrib-dependent and cadherin-mediated apoptosis. Curr. Biol. 2012, 22, 1747–1754. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, M.-B.; Christensen, S.T.; Hoffmann, E.K. Effects of osmotic stress on the activity of MAPKs and PDGFR-β-mediated signal transduction in NIH-3T3 fibroblasts. Am. J. Physiol. Cell Physiol. 2008, 294, C1046–C1055. [Google Scholar] [CrossRef]
- Messaoud, N.B.; Yue, J.; Valent, D.; Katzarova, I.; López, J.M. Osmostress-induced apoptosis in Xenopus oocytes: Role of stress protein kinases, calpains and Smac/DIABLO. PLoS ONE 2015, 10, e0124482. [Google Scholar]
- Yue, J.; Messaoud, N.B.; López, J.M. Hyperosmotic shock engages two positive feedback loops through Caspase-3-dependent Proteolysis of JNK1-2 and Bid. J. Biol. Chem. 2015, 290, 30375–30389. [Google Scholar] [CrossRef] [Green Version]
- Bevilacqua, E.; Wang, X.; Majumder, M.; Gaccioli, F.; Yuan, C.L.; Wang, C.; Zhu, X.; Jordan, L.E.; Scheuner, D.; Kaufman, R.J. eIF2α phosphorylation tips the balance to apoptosis during osmotic stress. J. Biol. Chem. 2010, 285, 17098–17111. [Google Scholar] [CrossRef] [Green Version]
- Najafi, A.; Adutwum, E.; Yari, A.; Salehi, E.; Mikaeili, S.; Dashtestani, F.; Abolhassani, F.; Rashki, L.; Shiasi, S.; Asadi, E. Melatonin affects membrane integrity, intracellular reactive oxygen species, caspase3 activity and AKT phosphorylation in frozen thawed human sperm. Cell Tissue Res. 2018, 372, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.-Y.; Li, Q.; Ren, F.; Xi, H.-M.; Lv, D.-L.; Li, Y.; Hu, J.-H. Melatonin protects goat spermatogonial stem cells against oxidative damage during cryopreservation by improving antioxidant capacity and inhibiting mitochondrial apoptosis pathway. Oxid. Med. Cell. Longev. 2020, 2020, 5954635. [Google Scholar] [CrossRef]
- García, J.J.; López-Pingarrón, L.; Almeida-Souza, P.; Tres, A.; Escudero, P.; García-Gil, F.A.; Tan, D.X.; Reiter, R.J.; Ramírez, J.M.; Bernal-Pérez, M. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: A review. J. Pineal Res. 2014, 56, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, F.; Betti, M.; Canonico, B.; Arcangeletti, M.; Ferri, P.; Galli, F.; Papa, S. ERK MAPK activation mediates the antiapoptotic signaling of melatonin in UVB-stressed U937 cells. Free Radic. Biol. Med. 2009, 46, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Ofosu, J.; Qazi, I.H.; Fang, Y.; Zhou, G. Use of melatonin in sperm cryopreservation of farm animals: A brief review. Anim. Reprod. Sci. 2021, 233, 106850. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, G.; Moro, N.; Ruggiero, F.M.; Paradies, G. Melatonin inhibits cardiolipin peroxidation in mitochondria and prevents the mitochondrial permeability transition and cytochrome c release. Free Radic. Biol. Med. 2009, 47, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Yang, Y.; Yi, W.; Yan, J.; Liang, Z.; Wang, N.; Li, Y.; Chen, W.; Yu, S.; Jin, Z. New role of JAK2/STAT3 signaling in endothelial cell oxidative stress injury and protective effect of melatonin. PLoS ONE 2013, 8, e57941. [Google Scholar] [CrossRef] [Green Version]
- Chetsawang, B.; Putthaprasart, C.; Phansuwan-Pujito, P.; Govitrapong, P. Melatonin protects against hydrogen peroxide-induced cell death signaling in SH-SY5Y cultured cells: Involvement of nuclear factor kappa B, Bax and Bcl-2. J. Pineal Res. 2006, 41, 116–123. [Google Scholar] [CrossRef]
- Kakkar, P.; Singh, B. Mitochondria: A hub of redox activities and cellular distress control. Mol. Cell. Biochem. 2007, 305, 235–253. [Google Scholar] [CrossRef]
- He, B.; Guo, H.; Gong, Y.; Zhao, R. Lipopolysaccharide-induced mitochondrial dysfunction in boar sperm is mediated by activation of oxidative phosphorylation. Theriogenology 2017, 87, 1–8. [Google Scholar] [CrossRef]
- Gonzalvez, F.; Pariselli, F.; Dupaigne, P.; Budihardjo, I.; Lutter, M.; Antonsson, B.; Diolez, P.; Manon, S.; Martinou, J.; Goubern, M. tBid interaction with cardiolipin primarily orchestrates mitochondrial dysfunctions and subsequently activates Bax and Bak. Cell Death Differ. 2005, 12, 614–626. [Google Scholar] [CrossRef] [Green Version]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, M.J.; Meyers, S.A. Antioxidant treatment in the absence of exogenous lipids and proteins protects rhesus macaque sperm from cryopreservation-induced cell membrane damage. Theriogenology 2011, 76, 168–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinehr, R.; Görg, B.; Becker, S.; Qvartskhava, N.; Bidmon, H.J.; Selbach, O.; Haas, H.L.; Schliess, F.; Häussinger, D. Hypoosmotic swelling and ammonia increase oxidative stress by NADPH oxidase in cultured astrocytes and vital brain slices. Glia 2007, 55, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.S.; Shkryl, V.M.; Nowycky, M.C.; Shirokova, N. Reactive oxygen species contribute to Ca2+ signals produced by osmotic stress in mouse skeletal muscle fibres. J. Physiol. 2008, 586, 197–210. [Google Scholar] [CrossRef] [PubMed]
- O‘Neil, R.G.; Heller, S. The mechanosensitive nature of TRPV channels. Pflüg. Arch. 2005, 451, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Tan, D.-x.; Manchester, L.C.; Qi, W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species. Cell Biochem. Biophys. 2001, 34, 237–256. [Google Scholar] [CrossRef]
- Li, B.; Zhang, H.; AKBAR, M.; KIM, H.-Y. Negative regulation of cytosolic phospholipase A2 by melatonin in the rat pineal gland. Biochem. J. 2000, 351, 709–716. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, S.; Zhao, X.; Wei, T. Melatonin impairs NADPH oxidase assembly and decreases superoxide anion production in microglia exposed to amyloid-β1–42. J. Pineal Res. 2008, 45, 157–165. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Zeng, W.; Wei, J. Edaravone protects against hyperosmolarity-induced oxidative stress and apoptosis in primary human corneal epithelial cells. PLoS ONE 2017, 12, e0174437. [Google Scholar] [CrossRef]
- Jiao, S.; Li, J.; Liu, B.; Yang, M.; Xiu, J.; Qu, D. Nucleus pulposus cell apoptosis is attenuated by CDMP-2 through regulating oxidative damage under the hyperosmotic environment. Biosci. Rep. 2018, 38, BSR20181176. [Google Scholar] [CrossRef] [Green Version]
- Morgan, M.J.; Liu, Z.-g. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoshan-Barmatz, V.; Krelin, Y.; Shteinfer-Kuzmine, A. VDAC1 functions in Ca2+ homeostasis and cell life and death in health and disease. Cell Calcium 2018, 69, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling. Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Gogvadze, V.; Robertson, J.D.; Enoksson, M.; Zhivotovsky, B.; Orrenius, S. Mitochondrial cytochrome c release may occur by volume-dependent mechanisms not involving permeability transition. Biochem. J. 2004, 378, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Mayer, B.; Oberbauer, R. Mitochondrial regulation of apoptosis. Physiology 2003, 18, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Burg, M.B. Response of renal inner medullary epithelial cells to osmotic stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 133, 661–666. [Google Scholar] [CrossRef]
- Desai, B.N.; Myers, B.R.; Schreiber, S.L. FKBP12-rapamycin-associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction. Proc. Natl. Acad. Sci. USA 2002, 99, 4319–4324. [Google Scholar] [CrossRef] [Green Version]
- Bambach, A.; Fernandes, M.P.; Ghosh, A.; Kruppa, M.; Alex, D.; Li, D.; Fonzi, W.A.; Chauhan, N.; Sun, N.; Agrellos, O.A. Goa1p of Candida albicans localizes to the mitochondria during stress and is required for mitochondrial function and virulence. Eukaryot. Cell 2009, 8, 1706–1720. [Google Scholar] [CrossRef] [Green Version]
- Pena, F.; Plaza Davila, M.; Ball, B.; Squires, E.; Martin Munoz, P.; Ortega Ferrusola, C.; Balao da Silva, C. The impact of reproductive technologies on stallion mitochondrial function. Reprod. Domest. Anim. 2015, 50, 529–537. [Google Scholar] [CrossRef]
- Martin, M.; Macias, M.; Escames, G.; Reiter, R.; Agapito, M.; Ortiz, G.; Acuña-Castroviejo, D. Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J. Pineal Res. 2000, 28, 242–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okatani, Y.; Wakatsuki, A.; Reiter, R.J. Melatonin protects hepatic mitochondrial respiratory chain activity in senescence-accelerated mice. J. Pineal Res. 2002, 32, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, Y.B.; Aydin, C.; Gorgisen, G. The effects of melatonin on oxidative stress and prevention of primordial follicle loss via activation of mTOR pathway in the rat ovary. Cell. Mol. Biol. 2017, 63, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Clapp-Lilly, K.L.; Smith, M.A.; Perry, G.; Harris, P.L.; Zhu, X.; Duffy, L.K. Melatonin acts as antioxidant and pro-oxidant in an organotypic slice culture model of Alzheimer’s disease. Neuroreport 2001, 12, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Girish, K.S.; Paul, M.; Thushara, R.M.; Hemshekhar, M.; Sundaram, M.S.; Rangappa, K.S.; Kemparaju, K. Melatonin elevates apoptosis in human platelets via ROS mediated mitochondrial damage. Biochem. Biophys. Res. Commun. 2013, 438, 198–204. [Google Scholar] [CrossRef]
- Bejarano, I.; Espino, J.; Barriga, C.; Reiter, R.J.; Pariente, J.A.; Rodríguez, A.B. Pro-oxidant effect of melatonin in tumour leucocytes: Relation with its cytotoxic and pro-apoptotic effects. Basic Clin. Pharmacol. Toxicol. 2011, 108, 14–20. [Google Scholar] [CrossRef]
- Radogna, F.; Paternoster, L.; De Nicola, M.; Cerella, C.; Ammendola, S.; Bedini, A.; Tarzia, G.; Aquilano, K.; Ciriolo, M.; Ghibelli, L. Rapid and transient stimulation of intracellular reactive oxygen species by melatonin in normal and tumor leukocytes. Toxicol. Appl. Pharmacol. 2009, 239, 37–45. [Google Scholar] [CrossRef]
- Fu, J.; Zhao, S.D.; Liu, H.J.; Yuan, Q.H.; Liu, S.M.; Zhang, Y.M.; Ling, E.A.; Hao, A.J. Melatonin promotes proliferation and differentiation of neural stem cells subjected to hypoxia in vitro. J. Pineal Res. 2011, 51, 104–112. [Google Scholar] [CrossRef]
- Cui, P.; Yu, M.; Peng, X.; Dong, L.; Yang, Z. Melatonin prevents human pancreatic carcinoma cell PANC-1-induced human umbilical vein endothelial cell proliferation and migration by inhibiting vascular endothelial growth factor expression. J. Pineal Res. 2012, 52, 236–243. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef]
- Marquez-Curtis, L.A.; Janowska-Wieczorek, A.; McGann, L.E.; Elliott, J.A. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology 2015, 71, 181–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, O.; Smith, A.; Cooper, P.; Shelton, R.; Scheven, B. The effects of cryopreservation on cells isolated from adipose, bone marrow and dental pulp tissues. Cryobiology 2014, 69, 342–347. [Google Scholar] [CrossRef] [PubMed]




| Bovine (n = 8) | Human (n = 3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Suspended Cells | Adherent Cells | Suspended Cells | Adherent Cells | ||||||
| Control | Melatonin | Control | Melatonin | Control | Melatonin | Control | Melatonin | ||
| mOsm | Viability | mOsm | Viability | ||||||
| 25 | 0.57 ± 0.07 c | 0.72 ± 0.05 fc | 0.48 ± 0.03 c | 0.67 ± 0.03 fc | 25 | 0.68 ± 0.02 c | 0.78 ± 0.03 ec | 0.66 ± 0.0.2 c | 0.76 ± 0.03 dc |
| 150 | 0.68 ± 0.03 c | 0.84 ± 0.05 fc | 0.66 ± 0.02 c | 0.89 ± 0.05 fb | 150 | 0.8 ± 0.02 c | 0.93 ± 0.01 f | 0.79 ± 0.01 c | 0.95 ± 0.01 f |
| 500 | 0.66 ± 0.02 c | 0.85 ± 0.05 fc | 0.63 ± 0.03 c | 0.90 ± 0.08 fb | 500 | 0.81 ± 0.01 c | 0.94 ± 0.02 f | 0.78 ± 0.03 c | 0.90 ± 0.02 eb |
| 1000 | 0.42 ± 0.03 c | 0.56 ± 0.03 fc | 0.34 ± 0.02 c | 0.49 ± 0.02 fc | 1000 | 0.65 ± 0.01 c | 0.76 ± 0.02 ec | 0.53 ± 0.03 c | 0.74 ± 0.02 fc |
| ROS Levels | ROS Levels | ||||||||
| 25 | 2.54 ± 0.06 c | 1.92 ± 0.08 fc | 2.63 ± 0.1 c | 2.06 ± 0.12 fc | 25 | 1.85 ± 0.05 c | 1.64 ± 0.03 dc | 1.65 ± 0.03 c | 1.43 ± 0.02 fc |
| 150 | 1.91 ± 0.03 c | 1.35 ± 0.04 fc | 1.57 ± 0.03 c | 1.23 ± 0.05 | 150 | 1.69 ± 0.09 c | 1.43 ± 0.03 ec | 1.44 ± 0.02 c | 1.25 ± 0.02 fc |
| 500 | 1.97 ± 0.13 c | 1.37 ± 0.07 fc | 1.89 ± 0.02 c | 1.40 ± 0.03 eb | 500 | 1.97 ± 0.02 c | 1.56 ± 0.03 fc | 1.70 ± 0.03 c | 1.20 ± 0.04 fc |
| 1000 | 2.51 ± 0.06 c | 1.87 ± 0.05 fc | 2.46 ± 0.08 c | 2.04 ± 0.1 dc | 1000 | 2.81 ± 0.05 c | 2.21 ± 0.3 fc | 2.66 ± 0.03 c | 2.11 ± 0.03 fc |
| Bovine (n = 8) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Suspended Cells | Adherent Cells | |||||||||||
| Control (Melatonin 0 mM) | Melatonin 0.001 mM | Melatonin 0.01 mM | Melatonin 0.1 mM | Melatonin 1 mM | Melatonin 10 mM | Control (Melatonin 0 mM) | Melatonin 0.001 mM | Melatonin 0.01 mM | Melatonin 0.1 mM | Melatonin 1 mM | Melatonin 10 mM | |
| mOsm | Viability | |||||||||||
| 25 | 0.50 ± 0.01 c | 0.63 ± 0.01 fc | 0.64 ± 0.05 fc | 0.65 ± 0.05 fc | 0.51 ± 0.05 c | 0.20 ± 0.01 fc | 0.54 ± 0.03 c | 0.57 ± 0.03 c | 0.64 ± 0.04 c | 0.70 ± 0.04 fc | 0.55 ± 0.03 c | 0.16 ± 0.02 fc |
| 150 | 0.66 ± 0.1 c | 0.70 ± 0.02 c | 0.76 ± 0.03 c | 0.85 ± 0.02 fc | 0.71 ± 0.01 c | 0.24 ± 0.02 fc | 0.66 ± 0.04 c | 0.69 ± 0.03 c | 0.76 ± 0.02 c | 0.89 ± 0.02 f | 0.75 ± 0.02 c | 0.20 ± 0.03 fc |
| 300 | 1 ± 0 | 1.01 ± 0.01 | 1.05 ± 0.01 | 1.09 ± 0.01 | 0.87 ± 0.02 e | 0.72 ± 0.02 f | 1 ± 0 | 1.01 ± 0.01 | 1.04 ± 0.01 | 1.09 ± 0.02 | 0.92 ± 0.04 | 0.36 ± 0.03 f |
| 500 | 0.65 ± 0.03 c | 0.66 ± 0.01 c | 0.68 ± 0.06 c | 0.83 ± 0.06 fc | 0.75 ± 0.05 dc | 0.67 ± 0.03 c | 0.63 ± 0.04 c | 0.67 ± 0.03 c | 0.74 ± 0.04 c | 0.90 ± 0.06 f | 0.74 ± 0.04 c | 0.33 ± 0.03 fc |
| 1000 | 0.42 ± 0.03 c | 0.36 ± 0.02 c | 0.42 ± 0.03 dc | 0.49 ± 0.03 fc | 0.37 ± 0.03 c | 0.19 ± 0.03 fc | 0.33 ± 0.02 c | 0.43 ± 0.02 c | 0.53 ± 0.02 c | 0.56 ± 0.01 dc | 0.45 ± 0.01 c | 0.25 ± 0.02 c |
| mOsm | ROS Levels | |||||||||||
| 25 | 2.45 ± 0.07 c | 2.22 ± 0.07 c | 1.82 ± 0.02 fc | 1.86 ± 0.08 fc | 2.06 ± 0.09 fc | 2.31 ± 0.05 c | 2.60 ± 0.05 c | 2.39 ± 0.09 c | 2.10 ± 0.10 ec | 2.04 ± 0.04 fc | 2.23 ± 0.12 c | 2.75 ± 0.29 c |
| 150 | 1.94 ± 0.04 c | 1.75 ± 0.06 c | 1.37 ± 0.07 fc | 1.35 ± 0.03 fb | 1.65 ± 0.01 c | 1.98 ± 0.04 c | 1.62 ± 0.02 c | 1.45 ± 0.07 a | 1.10 ± 0.02 e | 1.19 ± 0.02 | 1.41 ± 0.07 | 1.74 ± 0.12 c |
| 300 | 1 ± 0 | 0.90 ± 0.01 | 0.73 ± 0.02 | 0.78 ± 0.04 | 1.00 ± 0.06 | 1.38 ± 0.03 f | 1 ± 0 | 0.92 ± 0.02 | 0.74 ± 0.02 | 0.70 ± 0.03 | 0.90 ± 0.03 | 1.21 ± 0.02 |
| 500 | 2.01 ± 0.03 c | 1.78 ± 0.06 c | 1.39 ± 0.04 fc | 1.39 ± 0.02 fc | 1.79 ± 0.02 c | 1.96 ± 0.05 c | 1.92 ± 0.02 c | 1.73 ± 0.03 c | 1.43 ± 0.07 d | 1.48 ± 0.07 da | 1.67 ± 0.06 c | 1.92 ± 0.04 c |
| 1000 | 2.45 ± 0.03 c | 2.20 ± 0.08 c | 1.78 ± 0.06 fc | 1.86 ± 0.01 fc | 2.15 ± 0.01 dc | 2.35 ± 0.02 c | 2.47 ± 0.03 c | 2.24 ± 0.04 c | 1.93 ± 0.06 ec | 1.97 ± 0.02 ec | 2.16 ± 0.06 c | 2.43 ± 0.03 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asadi, E.; Najafi, A.; Benson, J.D. Exogenous Melatonin Ameliorates the Negative Effect of Osmotic Stress in Human and Bovine Ovarian Stromal Cells. Antioxidants 2022, 11, 1054. https://doi.org/10.3390/antiox11061054
Asadi E, Najafi A, Benson JD. Exogenous Melatonin Ameliorates the Negative Effect of Osmotic Stress in Human and Bovine Ovarian Stromal Cells. Antioxidants. 2022; 11(6):1054. https://doi.org/10.3390/antiox11061054
Chicago/Turabian StyleAsadi, Ebrahim, Atefeh Najafi, and James D Benson. 2022. "Exogenous Melatonin Ameliorates the Negative Effect of Osmotic Stress in Human and Bovine Ovarian Stromal Cells" Antioxidants 11, no. 6: 1054. https://doi.org/10.3390/antiox11061054
APA StyleAsadi, E., Najafi, A., & Benson, J. D. (2022). Exogenous Melatonin Ameliorates the Negative Effect of Osmotic Stress in Human and Bovine Ovarian Stromal Cells. Antioxidants, 11(6), 1054. https://doi.org/10.3390/antiox11061054