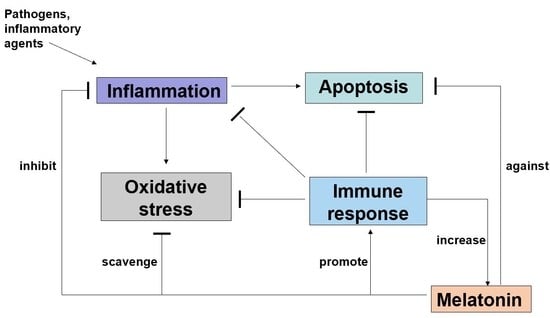
Insight of Melatonin: The Potential of Melatonin to Treat Bacteria-Induced Mastitis
Abstract
:1. Introduction
2. The Pathogenesis of Bacteria-Induced Mastitis
3. The Effect of Melatonin on Inflammation
4. The Effect of Melatonin on Bacteria-Induced Mastitis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cha, E.; Bar, D.; Hertl, J.A.; Tauer, L.W.; Bennett, G.; González, R.N.; Schukken, Y.H.; Welcome, F.L.; Gröhn, Y.T. The cost and management of different types of clinical mastitis in dairy cows estimated by dynamic programming. J. Dairy Sci. 2011, 94, 4476–4487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollin, E.; Dhuyvetter, K.C.; Overton, M.W. The cost of clinical mastitis in the first 30 days of lactation: An economic modeling tool. Prev. Vet. Med. 2015, 122, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahl, M.O.; De Vries, A.; Maunsell, F.P.; Galvao, K.N.; Risco, C.A.; Hernandez, J.A. Epidemiologic and economic analyses of pregnancy loss attributable to mastitis in primiparous Holstein cows. J. Dairy Sci. 2018, 101, 10142–10150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuenzalida, M.J.; Fricke, P.M.; Ruegg, P.L. The association between occurrence and severity of subclinical and clinical mastitis on pregnancies per artificial insemination at first service of Holstein cows. J. Dairy Sci. 2015, 98, 3791–3805. [Google Scholar] [CrossRef] [Green Version]
- Wellnitz, O.; Bruckmaier, R.M. The innate immune response of the bovine mammary gland to bacterial infection. Vet. J. 2012, 192, 148–152. [Google Scholar] [CrossRef]
- Dalanezi, F.M.; Joaquim, S.F.; Guimarães, F.F.; Guerra, S.T.; Lopes, B.C.; Schmidt, E.M.S.; Cerri, R.L.A.; Langoni, H. Influence of pathogens causing clinical mastitis on reproductive variables of dairy cows. J. Dairy Sci. 2020, 103, 3648–3655. [Google Scholar] [CrossRef]
- Saenz-de-Juano, M.D.; Silvestrelli, G.; Bauersachs, S.; Ulbrich, S.E. Determining extracellular vesicles properties and miRNA cargo variability in bovine milk from healthy cows and cows undergoing subclinical mastitis. BMC Genom. 2022, 23, 189. [Google Scholar] [CrossRef]
- Duarte, C.M.; Freitas, P.P.; Bexiga, R. Technological advances in bovine mastitis diagnosis: An overview. J. Vet. Diagn. Investig. 2015, 27, 665–672. [Google Scholar] [CrossRef] [Green Version]
- Mordmuang, A.; Brouillette, E.; Voravuthikunchai, S.P.; Malouin, F. Evaluation of a Rhodomyrtus tomentosa ethanolic extract for its therapeutic potential on Staphylococcus aureus infections using in vitro and in vivo models of mastitis. Vet. Res. 2019, 50, 49. [Google Scholar] [CrossRef] [Green Version]
- Procópio, T.F.; Moura, M.C.; Bento, E.F.L.; Soares, T.; Coelho, L.C.B.B.; Bezerra, R.P.; Mota, R.A.; Porto, A.L.F. Looking for alternative treatments for bovine and caprine mastitis: Evaluation of the potential of Calliandra surinamensis leaf pinnulae lectin (CasuL), both alone and in combination with antibiotics. Microbiologyopen 2019, 8, e869. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.Y.; Shi, X.C.; Laborda, P. Indole-based melatonin analogues: Synthetic approaches and biological activity. Eur. J. Med. Chem. 2020, 185, 111847. [Google Scholar] [CrossRef] [PubMed]
- Amaral, F.G.D.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, R.; Jungheim, E.S.; Fay, J.C.; Bates, K.; Herzog, E.D.; England, S.K. Riding the rhythm of melatonin through pregnancy to deliver on time. Front. Endocrinol. 2019, 10, 616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schomerus, C.; Korf, H.W. Mechanisms regulating melatonin synthesis in the mammalian pineal organ. Ann. N. Y. Acad. Sci. 2005, 1057, 372–383. [Google Scholar] [CrossRef]
- Laurent, V.; Sengupta, A.; Sánchez-Bretaño, A.; Hicks, D.; Tosini, G. Melatonin signaling affects the timing in the daily rhythm of phagocytic activity by the retinal pigment epithelium. Exp. Eye Res. 2017, 165, 90–95. [Google Scholar] [CrossRef]
- Slominski, A.T.; Semak, I.; Fischer, T.W.; Kim, T.K.; Kleszczyński, K.; Hardeland, R.; Reiter, R.J. Metabolism of melatonin in the skin: Why is it important? Exp. Dermatol. 2017, 26, 563–568. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Reiter, R.J.; Davoodabadi, A.; Asemi, Z. Melatonin as a potential inhibitor of colorectal cancer: Molecular mechanisms. J. Cell. Biochem. 2019, 120, 12216–12223. [Google Scholar] [CrossRef]
- Cruz-Chamorro, I.; Álvarez-Sánchez, N.; Escalante-Andicoechea, C.; Carrillo-Vico, A.; Rubio, A. Temporal expression patterns of the melatoninergic system in the human thymus of children. Mol. Metab. 2019, 28, 83–90. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Calvo, J.R.; Abreu, P.; Hong, H.S. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: Possible role as intracrine, autocrine, and/or paracrine substance. FASEB J. 2004, 18, 537–539. [Google Scholar] [CrossRef]
- Pedrosa, A.M.; Weinlich, R.; Mognol, G.P.; Robbs, B.K.; Viola, J.P.; Campa, A.; Amarante-Mendes, G.P. Melatonin protects CD4+ T cells from activation-induced cell death by blocking NFAT-mediated CD95 ligand upregulation. J. Immunol. 2010, 184, 3487–3494. [Google Scholar] [CrossRef] [Green Version]
- Cardinali, D.P.; Ladizesky, M.G.; Boggio, V.; Cutrera, R.A.; Mautalen, C. Melatonin effects on bone: Experimental facts and clinical perspectives. J. Pineal Res. 2003, 34, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Amaral, F.G. Melatonin as a hormone: New physiological and clinical insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, T.L.; Choo, F.N.; Tan, S.M. The efficacy of melatonin and melatonin agonists in insomnia—An umbrella review. J. Psychiatr. Res. 2020, 121, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Parry, B.L.; Meliska, C.J.; Lopez, A.M.; Sorenson, D.L.; Martinez, L.F.; Orff, H.J.; Hauger, R.L.; Kripke, D.F. Early versus late wake therapy improves mood more in antepartum versus postpartum depression by differentially altering melatonin-sleep timing disturbances. J. Affect. Disord. 2019, 245, 608–616. [Google Scholar] [CrossRef] [Green Version]
- Lewandowska, K.; Małkiewicz, M.A.; Siemiński, M.; Cubała, W.J.; Winklewski, P.J. The role of melatonin and melatonin receptor agonist in the prevention of sleep disturbances and delirium in intensive care unit—A clinical review. Sleep Med. 2020, 69, 127–134. [Google Scholar] [CrossRef]
- Stepicheva, N.A.; Weiss, J.; Peng, S.; Yazdankhah, M.; Ghosh, S.; Bhutto, I.A.; Hose, S.; Zigler, J.S.; Sinha, D. Melatonin as the possible link between age-related retinal regeneration and the disrupted circadian rhythm in elderly. Adv. Exp. Med. Biol. 2019, 1185, 45–49. [Google Scholar]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Lee, L.; Tamura, I.; Maekawa, R.; Aasada, H. Melatonin and female reproduction. J. Obstet. Gynaecol. Res. 2014, 40, 1–11. [Google Scholar] [CrossRef]
- Cavalcante, B.N.; Matos-Brito, B.G.; Paulino, L.R.F.M.; Silva, B.R.; Aguiar, A.W.M.; de Almeida, E.F.M.; Souza, A.L.P.; Vasconcelos, G.L.; De Assis, E.I.T.; Silva, A.W.B.; et al. Effects of melatonin on morphology and development of primordial follicles during in vitro culture of bovine ovarian tissue. Reprod. Domest. Anim. 2019, 54, 1567–1573. [Google Scholar] [CrossRef]
- De, R.F.; Garcia-Ispierto, I.; López-Gatius, F. Seasonal heat stress: Clinical implications and hormone treatments for the fertility of dairy cows. Theriogenology 2015, 84, 659–666. [Google Scholar]
- El-Raey, M.; Geshi, M.; Somfai, T.; Kaneda, M.; Hirako, M.; Abdel-Ghaffar, A.E.; Sosa, G.A.; El-Roos, M.E.; Nagai, T. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol. Reprod. Dev. 2011, 78, 250–262. [Google Scholar] [CrossRef]
- Xu, X.F.; Wang, G.Q.; Ai, L.L.; Shi, J.; Zhang, J.; Chen, Y.X. Melatonin suppresses TLR9-triggered proinflammatory cytokine production in macrophages by inhibiting ERK1/2 and AKT activation. Sci. Rep. 2018, 8, 15579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markus, R.P.; Fernandes, P.A.; Kinker, G.S.; da Silveira Cruz-Machado, S.; Marçola, M. Immune-pineal axis—Acute inflammatory responses coordinate melatonin synthesis by pinealocytes and phagocytes. Br. J. Pharmacol. 2018, 175, 3239–3250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradkhani, F.; Moloudizargari, M.; Fallah, M.; Asghari, N.; Heidari Khoei, H.; Asghari, M.H. Immunoregulatory role of melatonin in cancer. J. Cell. Physiol. 2020, 235, 745–757. [Google Scholar] [CrossRef]
- Zhao, D.K.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, L.; Farokhi-Sisakht, F.; Badalzadeh, R.; Khabbaz, A.; Mahmoudi, J.; Sadigh-Eteghad, S. Nicotinamide mononucleotide and melatonin alleviate aging-induced cognitive impairment via modulation of mitochondrial function and apoptosis in the prefrontal cortex and hippocampus. Neuroscience 2019, 423, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Tung, Y.T.; Chen, H.Y.; Chiang, H.Y.; Hong, H.C.; Huang, K.C.; Hsu, S.P.; Huang, T.C.; Hsia, S.M. Melatonin activates cell death programs for the suppression of uterine leiomyoma cell proliferation. J. Pineal Res. 2020, 68, e12620. [Google Scholar] [CrossRef] [PubMed]
- Zubidat, A.E.; Fares, B.; Fares, F.; Haim, A. Artificial light at night of different spectral compositions differentially affects tumor growth in mice: Interaction with melatonin and epigenetic pathways. Cancer Control 2018, 25, 1073274818812908. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhu, Y.; Zhu, H.; Cai, R.; Wang, K.F.; Song, J.; Wang, R.X.; Zhou, R.X. Role of transforming growth factor β1 in the inhibition of gastric cancer cell proliferation by melatonin in vitro and in vivo. Oncol. Rep. 2019, 42, 753–762. [Google Scholar] [CrossRef]
- Hossain, M.; Uddin, M.S.; Uddin, G.M.S.; Sumsuzzman, D.M.; Islam, M.S.; Barreto, G.E.; Mathew, B.; Ashraf, G.M. Melatonin in alzheimer’s disease: A latent endogenous regulator of neurogenesis to mitigate alzheimer’s neuropathology. Mol. Neurobiol. 2019, 56, 8255–8276. [Google Scholar] [CrossRef]
- Yanar, K.; Simsek, B.; Çakatay, U. Integration of melatonin related redox homeostasis, aging, and circadian rhythm. Rejuvenation Res. 2019, 22, 409–419. [Google Scholar] [CrossRef]
- Owino, S.; Buonfiglio, D.D.C.; Tchio, C.; Tosini, G. Melatonin signaling a key regulator of glucose homeostasis and energy metabolism. Front. Endocrinol. 2019, 10, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Xia, H.Z.; Zhang, L.; Zhang, H.; Wang, D.; Tao, X. Protective effects of melatonin on sepsis-induced liver injury and dysregulation of gluconeogenesis in rats through activating SIRT1/STAT3 pathway. Biomed. Pharmacother. 2019, 117, 109150. [Google Scholar] [CrossRef] [PubMed]
- Trufakin, V.A.; Shurlygina, A.V.; Dushkin, M.I.; Khrapova, M.V.; Michurina, S.V.; Mel’nikova, E.V.; Panteleeva, N.G.; Tenditnik, M.V. Effect of melatonin on cellular composition of the spleen and parameters of lipid metabolism in rats with alimentary obesity. Bull. Exp. Biol. Med. 2014, 158, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.Q.; Yu, W.S.; Wei, W.; Zhang, X.; Tian, Y.; Sherif, M.; Liu, X.; Dong, C.; Wu, W.; Zhang, L.; et al. Melatonin reduces intramuscular fat deposition by promoting lipolysis and increasing mitochondrial function. J. Lipid Res. 2019, 60, 767–782. [Google Scholar] [CrossRef]
- Brencher, L.; Oude, L.M.; Effenberger-Neidnicht, K. Administration of exogenous melatonin after the onset of systemic inflammation is hardly beneficial. Inflammation 2017, 40, 1672–1677. [Google Scholar] [CrossRef]
- Yilmaz, B.; Kilic, S.; Aksakal, O.; Ertas, I.E.; Tanrisever, G.G.; Aksoy, Y.; Lortlar, N.; Kelekci, S.; Gungor, T. Melatonin causes regression of endometriotic implants in rats by modulating angiogenesis, tissue levels of antioxidants and matrix metalloproteinases. Arch. Gynecol. Obstet. 2015, 292, 209–216. [Google Scholar] [CrossRef]
- Doğanlar, Z.B.; Güçlü, H.; Öztopuz, Ö.; Türkön, H.; Dogan, A.; Uzun, M.; Doğanlar, O. The role of melatonin in oxidative stress, DNA damage, apoptosis and angiogenesis in fetal eye under preeclampsia and melatonin deficiency stress. Curr. Eye Res. 2019, 44, 1157–1169. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Pilar Terron, M.; Flores, L.J.; Koppisepi, S. Medical implications of melatonin: Receptor-mediated and receptor-independent actions. Adv. Med. Sci. 2007, 52, 11–28. [Google Scholar]
- Pechanova, O.; Paulis, L.; Simko, F. Peripheral and central effects of melatonin on blood pressure regulation. Int. J. Mol. Sci. 2014, 15, 17920–17937. [Google Scholar] [CrossRef] [Green Version]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef] [Green Version]
- Juszczak, M.; Wolak, M.; Bojanowska, E.; Piera, L.; Roszczyk, M. The role of melatonin membrane receptors in melatonin-dependent oxytocin secretion from the rat hypothalamo-neurohypophysial system-an in vitro and in vivo approach. Endokrynol. Pol. 2016, 67, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Mayo, J.C.; Sainz, R.M.; González-Menéndez, P.; Hevia, D.; Cernuda-Cernuda, R. Melatonin transport into mitochondria. Cell. Mol. Life Sci. 2017, 74, 3927–3940. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, D.D.; Zhu, P.J.; Ma, Q.; Toan, S.; Wang, J.; Hu, S.; Chen, Y.; Zhang, Y. Inhibitory effect of melatonin on necroptosis via repressing the Ripk3-PGAM5-CypD-mPTP pathway attenuates cardiac microvascular ischemia-reperfusion injury. J. Pineal Res. 2018, 65, e12503. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Xu, H.; Liu, C.B.; Wei, Z.; Wang, Z.; Zhao, L.; Ren, L. Melatonin ameliorates endoplasmic reticulum stress in N2a neuroblastoma cell hypoxia-reoxygenation injury by activating the AMPK-Pak2 pathway. Cell Stress Chaperones 2019, 24, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin, hormone of darkness and more: Occurrence, control mechanisms, actions and bioactive metabolites. Cell. Mol. Life Sci. 2008, 65, 2001–2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardeland, R. Melatonin and the electron transport chain. Cell. Mol. Life Sci. 2017, 74, 3883–3896. [Google Scholar] [CrossRef]
- Boutin, J.A.; Ferry, G. Is there sufficient evidence that the melatonin binding site is quinone reductase 2? J. Pharmacol. Exp. Ther. 2019, 368, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Menéndez-Menéndez, J.; Martínez-Campa, C. Melatonin: An anti-tumor agent in hormone-dependent cancers. Int. J. Endocrinol. 2018, 2018, 3271948. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin: Signaling mechanisms of a pleiotropic agent. Biofactors 2009, 35, 183–192. [Google Scholar] [CrossRef]
- Smith, G.L.; Friggens, N.C.; Ashworth, C.J.; Chagunda, M.G.G. Association between body energy content in the dry period and post-calving production disease status in dairy cattle. Animal 2017, 11, 1590–1598. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.H.C.; Hötzel, M.J.; Longo, C.; Cardoso, C.S.; Costa, J.H. A survey of management practices that influence production and welfare of dairy cattle on family farms in southern Brazil. J. Dairy Sci. 2013, 96, 307–317. [Google Scholar] [CrossRef]
- Supa-Amornkul, S.; Mongkolsuk, P.; Summpunn, P.; Chaiyakunvat, P.; Navaratdusit, W.; Jiarpinitnun, C.; Chaturongakul, S. Staphylococcus aureus alternative sigma factor B in bovine mastitis-causing: Characterization of its role in biofilm formation, resistance to hydrogen peroxide stress, regulon members. Front. Microbiol. 2019, 10, 2493. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Murakami, H.; Kakinuma, S.; Murao, K.; Ohmae, K.; Isobe, N.; Akamatsu, H.; Seto, T.; Hashimura, S.; Konda, K.; et al. Association between bovine leukemia virus proviral load and severity of clinical mastitis. J. Vet. Med. Sci. 2019, 81, 1431–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelopoulou, A.; Field, D.; Ryan, C.A.; Stanton, C.; Hill, C.; Ross, R.P. The microbiology and treatment of human mastitis. Med. Microbiol. Immunol. 2018, 207, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Marbach, H.; Mayer, K.; Vogl, C.; Lee, J.Y.H.; Monk, I.R.; Sordelli, D.O.; Buzzola, F.R.; Ehling-Schulz, M.; Grunert, T. Within-host evolution of bovine Staphylococcus aureus selects for a SigB-deficient pathotype characterized by reduced virulence but enhanced proteolytic activity and biofilm formation. Sci. Rep. 2019, 9, 13479. [Google Scholar] [CrossRef] [Green Version]
- Dalen, G.; Rachah, A.; Nørstebø, H.; Schukken, Y.H.; Reksen, O. Dynamics of somatic cell count patterns as a proxy for transmission of mastitis pathogens. J. Dairy Sci. 2019, 102, 11349–11358. [Google Scholar] [CrossRef]
- Tekbas, O.F.; Ogur, R.; Korkmaz, A.; Kilic, A.; Reiter, R.J. Melatonin as an antibiotic: New insights into the actions of this ubiquitous molecule. J. Pineal Res. 2008, 44, 222–226. [Google Scholar] [CrossRef]
- Rocha, L.S.; Silva, D.M.; Silva, M.P.; Vidigal, P.M.P.; Silva, J.C.F.; Guerra, S.T.; Ribeiro, M.G.; Mendes, T.A.O.; Ribon, A.O.B. Comparative genomics of Staphylococcus aureus associated with subclinical and clinical bovine mastitis. PLoS ONE 2019, 14, e0220804. [Google Scholar] [CrossRef] [Green Version]
- Côté-Gravel, J.; Malouin, F. Symposium review: Features of Staphylococcus aureus mastitis pathogenesis that guide vaccine development strategies. J. Dairy Sci. 2019, 102, 4727–4740. [Google Scholar] [CrossRef]
- Kamaruzzaman, N.F.; Chong, S.Q.Y.; Edmondson-Brown, K.M.; Ntow-Boahene, W.; Bardiau, M.; Good, L. Bactericidal and anti-biofilm effects of polyhexamethylene biguanide in models of intracellular and biofilm of Staphylococcus aureus isolated from bovine mastitis. Front. Microbiol. 2017, 8, 1518. [Google Scholar] [CrossRef]
- Ma, S.Y.; Tong, C.; Ibeagha-Awemu, E.M.; Zhao, X. Identification and characterization of differentially expressed exosomal microRNAs in bovine milk infected with Staphylococcus aureus. BMC Genom. 2019, 20, 934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.H.; Li, J.; Qiao, M.F.; Meng, D.; Meng, Q.; Qiao, J.; Zhang, X.; Wang, L.; Cai, K.; Zhang, J.; et al. Staphylococcus aureus characteristic profiles of biofilm, enterotoxins and virulence of isolates from dairy cows in Xinjiang Province, China. J. Vet. Sci. 2019, 20, e74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naqvi, S.A.; De, B.J.; Dufour, S.; Barkema, H.W. Udder health in Canadian dairy heifers during early lactation. J. Dairy Sci. 2018, 101, 3233–3247. [Google Scholar] [CrossRef] [PubMed]
- Fogsgaard, K.K.; Løvendahl, P.; Bennedsgaard, T.W.; Østergaard, S. Changes in milk yield, lactate dehydrogenase, milking frequency, and interquarter yield ratio persist for up to 8 weeks after antibiotic treatment of mastitis. J. Dairy Sci. 2015, 98, 7686–7698. [Google Scholar] [CrossRef] [PubMed]
- Fuenzalida, M.J.; Ruegg, P.L. Short communication: Longitudinal study of quarter-level somatic cell responses after naturally occurring, nonsevere clinical mastitis diagnosed as culture negative, or caused by Escherichia coli or Klebsiella pneumoniae, and randomly assigned to a no-treatment group or to receive intramammary ceftiofur. J. Dairy Sci. 2019, 102, 11476–11482. [Google Scholar] [PubMed] [Green Version]
- Fuenzalida, M.J.; Ruegg, P.L. Negatively controlled, randomized clinical trial to evaluate intramammary treatment of nonsevere, gram-negative clinical mastitis. J. Dairy Sci. 2019, 102, 5438–5457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wall, S.K.; Wellnitz, O.; Bruckmaier, R.M.; Schwarz, D. Differential somatic cell count in milk before, during, and after lipopolysaccharide- and lipoteichoic-acid-induced mastitis in dairy cows. J. Dairy Sci. 2018, 101, 5362–5373. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.K.; Chen, H.; Liu, H.M.; Cai, J.; Meng, L.; Dong, L.; Zheng, N.; Wang, J.; Wang, C. Streptococcus agalactiae quantitative polymerase chain reaction coupled with sodium dodecyl sulfate and propidium monoazide for detection of viable in milk. Front. Microbiol. 2019, 10, 661. [Google Scholar] [CrossRef]
- Sener, G.; Tuğtepe, H.; Velioğlu-Oğünç, A.; Cetinel, S.; Gedik, N.; Yeğen, B.C. Melatonin prevents neutrophil-mediated oxidative injury in Escherichia coli-induced pyelonephritis in rats. J. Pineal Res. 2006, 41, 220–227. [Google Scholar] [CrossRef]
- Shome, B.R.; Bhuvana, M.; Mitra, S.D.; Krithiga, N.; Shome, R.; Velu, D.; Banerjee, A.; Barbuddhe, S.B.; Prabhudas, K.; Rahman, H. Molecular characterization of Streptococcus agalactiae and Streptococcus uberis isolates from bovine milk. Trop. Anim. Health Prod. 2012, 44, 1981–1992. [Google Scholar] [CrossRef]
- Swanson, K.M.; Stelwagen, K.; Dobson, J.; Henderson, H.V.; Davis, S.R.; Farr, V.C.; Singh, K. Transcriptome profiling of Streptococcus uberis-induced mastitis reveals fundamental differences between immune gene expression in the mammary gland and in a primary cell culture model. J. Dairy Sci. 2009, 92, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Nedić, S.; Vakanjac, S.; Samardžija, M.; Borozan, S. Paraoxonase 1 in bovine milk and blood as marker of subclinical mastitis caused by Staphylococcus aureus. Res. Vet. Sci. 2019, 125, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Turk, R.; Piras, C.; Kovačić, M.; Samardžija, M.; Ahmed, H.; De Canio, M.; Urbani, A.; Meštrić, Z.F.; Soggiu, A.; Bonizzi, L.; et al. Proteomics of inflammatory and oxidative stress response in cows with subclinical and clinical mastitis. J. Proteom. 2012, 75, 4412–4428. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, Y.Q.; Zhu, Q.C.; Zang, H.Z.; Cai, J.; Wang, J.Q.; Cui, L.Y.; Meng, X.; Zhu, G.Q.; Li, J.J. Staphylococcus aureus induces autophagy in bovine mammary epithelial cells and the formation of autophagosomes facilitates intracellular replication of Staph. aureus. J. Dairy Sci. 2019, 102, 8264–8272. [Google Scholar] [CrossRef]
- Gao, W.; Li, X.; Liu, Z.H.; Fu, W.; Sun, Y.H.; Cao, W.H.; Tong, L.L.; Tang, B. A redox-responsive self-assembled nanoprobe for photoacoustic inflammation imaging to assess atherosclerotic plaque vulnerability. Anal. Chem. 2019, 91, 1150–1156. [Google Scholar] [CrossRef]
- Strobel, M.; Pförtner, H.; Tuchscherr, L.; Völker, U.; Schmidt, F.; Kramko, N.; Schnittler, H.J.; Fraunholz, M.J.; Löffler, B.; Peters, G.; et al. Post-invasion events after infection with Staphylococcus aureus are strongly dependent on both the host cell type and the infecting S. aureus strain. Clin. Microbiol. Infect. 2016, 22, 799–809. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, H.B.; Huang, W.L.; Qian, H.; Li, Y. TNF-α inhibitors with anti-oxidative stress activity from natural products. Curr. Top. Med. Chem. 2012, 12, 1408–1421. [Google Scholar] [CrossRef]
- Jin, S.H. The cross-regulation between autophagy and type I interferon signaling in host defense. Adv. Exp. Med. Biol. 2019, 1209, 125–144. [Google Scholar]
- Ogawa, M.; Matsuda, R.; Takada, N.; Tomokiyo, M.; Yamamoto, S.; Shizukuishi, S.; Yamaji, T.; Yoshikawa, Y.; Yoshida, M.; Tanida, I.; et al. Molecular mechanisms of Streptococcus pneumoniae-targeted autophagy via pneumolysin, Golgi-resident Rab41, and Nedd4-1-mediated K63-linked ubiquitination. Cell. Microbiol. 2018, 20, e12846. [Google Scholar] [CrossRef]
- Monkkonen, T.; Debnath, J. Inflammatory signaling cascades and autophagy in cancer. Autophagy 2018, 14, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Diaz, B.G.; Guizzardi, S.; Moine, L.; Tolosa de Talamoni, N. Oxidative stress, antioxidants and intestinal calcium absorption. World J. Gastroenterol. 2017, 23, 2841–2853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, F.; Chen, Z.; Su, Q.; Yan, M.; Zhang, Q.; Tan, J.; Qian, L.; Han, Y. Melatonin modulates IL-1β-induced extracellular matrix remodeling in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration and inflammation. Aging 2019, 11, 10499–10512. [Google Scholar] [CrossRef]
- Mortezaee, K.; Potes, Y.; Mirtavoos-Mahyari, H.; Motevaseli, E.; Shabeeb, D.; Musa, A.E.; Najafi, M.; Farhood, B. Boosting immune system against cancer by melatonin: A mechanistic viewpoint. Life Sci. 2019, 238, 116960. [Google Scholar] [CrossRef] [PubMed]
- Paterniti, I.; Cordaro, M.; Esposito, E.; Cuzzocrea, S. The antioxidative property of melatonin against brain ischemia. Expert Rev. Neurother. 2016, 16, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Deng, C.; Ma, Z.Q.; Wang, D.; Fan, C.; Li, T.; Di, S.; Gong, B.; Reiter, R.J.; Yang, Y. Utilizing melatonin to combat bacterial infections and septic injury. Br. J. Pharmacol. 2017, 174, 754–768. [Google Scholar] [CrossRef]
- Hardeland, R. Taxon- and site-specific melatonin catabolism. Molecules 2017, 22, 12015. [Google Scholar] [CrossRef] [Green Version]
- Abadi, S.H.M.H.; Shirazi, A.; Alizadeh, A.M.; Changizi, V.; Najafi, M.; Khalighfard, S.; Nosrati, H. The effect of melatonin on superoxide dismutase and glutathione peroxidase activity, and malondialdehyde levels in the targeted and the non-targeted lung and heart tissues after irradiation in xenograft mice colon cancer. Curr. Mol. Pharmacol. 2018, 11, 326–335. [Google Scholar] [CrossRef]
- Anderson, G.; Maes, M.; Markus, R.P.; Rodriguez, M. Ebola virus: Melatonin as a readily available treatment option. J. Med. Virol. 2015, 87, 537–543. [Google Scholar] [CrossRef]
- Srinivasan, V.; Pandi-Perumal, S.R.; Spence, D.W.; Kato, H.; Cardinali, D.P. Melatonin in septic shock: Some recent concepts. J. Crit. Care 2010, 25, 656.e1–656.e6. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.X.; Zhao, L.F.; Tao, J.J.; Li, L. Protective role of melatonin in early-stage and end-stage liver cirrhosis. J. Cell. Mol. Med. 2019, 23, 7151–7162. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, A.; Arabacı Tamer, S.; Sahin, D.; Bagriacik, F.; Kahraman, M.M.; Onur, N.D.; Cayirli, Y.B.; Cilingir Kaya, Ö.T.; Aksu, B.; Akdeniz, E.; et al. The effects of antibiotics and melatonin on hepato-intestinal inflammation and gut microbial dysbiosis induced by a short-term high-fat diet consumption in rats. Br. J. Nutr. 2019, 122, 841–855. [Google Scholar] [CrossRef]
- Mannino, G.; Caradonna, F.; Cruciata, I.; Lauria, A.; Perrone, A.; Gentile, C. Melatonin reduces inflammatory response in human intestinal epithelial cells stimulated by interleukin-1β. J. Pineal Res. 2019, 67, e12598. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.M.; Kubota, H.; Okita, M.; Maeda, T. The anti-inflammatory and antioxidant effects of melatonin on LPS-stimulated bovine mammary epithelial cells. PLoS ONE 2017, 12, e0178525. [Google Scholar] [CrossRef] [PubMed]
- Arioz, B.; Tastan, B.; Tarakcioglu, E.; Tufekci, K.U.; Olcum, M.; Ersoy, N.; Bagriyanik, A.; Genc, K.; Genc, S. Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Front. Immunol. 2019, 10, 1511. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.X.; Dong, Y.L.; Cao, J.; Lin, R.; Wang, X.; Yu, Z.; Chen, Y. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J. Pineal Res. 2019, 67, e12574. [Google Scholar] [CrossRef] [PubMed]
- Nasheed Hamad Almohammed, Z.; Moghani-Ghoroghi, F.; Ragerdi-Kashani, I.; Fathi, R.; Tahaei, L.S.; Naji, M.; Pasbakhsh, P. The effect of melatonin on mitochondrial function and autophagy in in vitro matured oocytes of aged mice. Cell J. 2020, 22, 9–16. [Google Scholar]
- Michan, S.; Sinclair, D. Sirtuins in mammals: Insights into their biological function. Biochem. J. 2007, 404, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Otsuka, R.; Hayano, K.; Matsubara, H. Role of sirtuins in esophageal cancer: Current status and future prospects. World J. Gastrointest. Oncol. 2022, 14, 794–807. [Google Scholar] [CrossRef]
- Ahn, B.H.; Kim, H.S.; Song, S.; Lee, I.H.; Liu, J.; Vassilopoulos, A.; Deng, C.X. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. USA 2008, 105, 14447–14452. [Google Scholar] [CrossRef] [Green Version]
- Jung-Hynes, B.; Schmit, T.L.; Reagan-Shaw, S.R.; Siddiqui, I.A.; Mukhtar, H.; Ahmad, N. Melatonin, a novel Sirt1 inhibitor, imparts antiproliferative effects against prostate cancer in vitro in culture and in vivo in TRAMP model. J. Pineal Res. 2011, 50, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Areti, A.; Komirishetty, P.; Akuthota, M.; Malik, R.A.; Kumar, A. Melatonin prevents mitochondrial dysfunction and promotes neuroprotection by inducing autophagy during oxaliplatin-evoked peripheral neuropathy. J. Pineal Res. 2017, 62, e12393. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Li, Y.Y.; Cui, W.; Li, L.B.; Zhang, Z.C.; Tian, B.P.; Zhang, G.S. Melatonin Attenuates Pain Hypersensitivity and Decreases Astrocyte-Mediated Spinal Neuroinflammation in a Rat Model of Oxaliplatin-Induced Pain. Inflammation 2017, 40, 2052–2061. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Tae, H.J.; Kim, I.S.; Song, M.; Kim, H.; Lee, T.K.; Kim, Y.M.; Ryoo, S.; Kim, D.W.; Lee, C.H.; et al. Melatonin alleviates asphyxial cardiac arrest-induced cerebellar Purkinje cell death by attenuation of oxidative stress. Exp. Neurol. 2019, 320, 112983. [Google Scholar] [CrossRef]
- Li, Y.L.; Guo, Y.; Fan, Y.; Tian, H.; Li, K.; Mei, X. Melatonin enhances autophagy and reduces apoptosis to promote locomotor recovery in spinal cord injury via the PI3K/AKT/mTOR signaling pathway. Neurochem. Res. 2019, 44, 2007–2019. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Peng, W.; Yin, S.; Zhao, J.; Fu, B.; Zhang, J.; Mao, T.; Wu, H.; Zhang, Y. Melatonin improves age-induced fertility decline and attenuates ovarian mitochondrial oxidative stress in mice. Sci. Rep. 2016, 6, 35165. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc: An antioxidant and anti-inflammatory agent: Role of zinc in degenerative disorders of aging. J. Trace Elem. Med. Biol. 2014, 28, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension what is the clinical significance? Hypertension 2004, 44, 248–252. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G. Breast cancer: Occluded role of mitochondria N-acetylserotonin/melatonin ratio in coordinating pathophysiology. Biochem. Pharmacol. 2019, 168, 259–268. [Google Scholar] [CrossRef]
- De Castro, T.B.; Bordin-Junior, N.A.; de Almeida, E.A.; de Campos Zuccari, D.A.P. Evaluation of melatonin and AFMK levels in women with breast cancer. Endocrine 2018, 62, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Kubatka, P.; Zubor, P.; Busselberg, D.; Kwon, T.K.; Adamek, M.; Petrovic, D.; Opatrilova, R.; Gazdikova, K.; Caprnda, M.; Rodrigo, L.; et al. Melatonin and breast cancer: Evidences from preclinical and human studies. Crit. Rev. Oncol. Hematol. 2018, 122, 133–143. [Google Scholar] [CrossRef]
- Rowe, S.E.; Wagner, N.J.; Li, L.; Beam, J.E.; Wilkinson, A.D.; Radlinski, L.C.; Zhang, Q.; Miao, E.A.; Conlon, B.P. Reactive oxygen species induce antibiotic tolerance during systemic Staphylococcus aureus infection. Nat. Microbiol. 2020, 5, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, V.; Chalkias, A.; Georgiou, G.; Papalois, A.; Kouskouni, E.; Baka, S.; Xanthos, T. Initial immune response in Escherichia coli, Staphylococcus aureus, and Candida albicans bacteremia. Inflammation 2020, 43, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.N.; Wang, P.; Mao, Y.M.; Dan, Y.L.; Wu, Q.; Li, X.M.; Wang, D.G.; Davis, C.; Hu, W.; Pan, H.F. Potential role of melatonin in autoimmune diseases. Cytokine Growth Factor Rev. 2019, 48, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Zhang, J.; Reiter, R.J.; Ma, X. Melatonin mediates mucosal immune cells, microbial metabolism, and rhythm crosstalk: A therapeutic target to reduce intestinal inflammation. Med. Res. Rev. 2020, 40, 606–632. [Google Scholar] [CrossRef]
- Perfilyeva, Y.V.; Ostapchuk, Y.O.; Abdolla, N.; Tleulieva, R.; Krasnoshtanov, V.C.; Belyaev, N.N. Exogenous melatonin up-regulates expression of CD62L by lymphocytes in aged mice under inflammatory and non-inflammatory conditions. Immunol. Investig. 2019, 48, 632–643. [Google Scholar] [CrossRef]
- Xia, Y.Y.; Chen, S.Y.; Zeng, S.J.; Zhao, Y.; Zhu, C.; Deng, B.; Zhu, G.; Yin, Y.; Wang, W.; Hardeland, R.; et al. Melatonin in macrophage biology: Current understanding and future perspectives. J. Pineal Res. 2019, 66, e12547. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.K.; Liu, G.; Chen, S.; Yin, J.; Wang, J.; Tan, B.; Wu, G.Y.; Bazer, F.W.; Peng, Y.Y.; Li, T.J.; et al. Melatonin signaling in T cells: Functions and applications. J. Pineal Res. 2017, 62, e12394. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, S.; Pfaffl, M.W.; Meyer, H.H.; Bruckmaier, R.M. Short-term changes of mRNA expression of various inflammatory factors and milk proteins in mammary tissue during LPS-induced mastitis. Domest. Anim. Endocrinol. 2004, 26, 111–126. [Google Scholar] [CrossRef]
- Chow, J.C.; Young, D.W.; Golenbock, D.T.; Christ, W.J.; Gusovsky, F. Toll-like receptor-4 mediates lipopolysac-charide-induced signal transduction. J. Biol. Chem. 1999, 274, 10689–10692. [Google Scholar] [CrossRef] [Green Version]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [Green Version]
- Pareek, R.; Wellnitz, O.; van Dorp, R.; Burton, J.; Kerr, D. Immunorelevant gene expression in LPS-challenged bovine mammary epithelial cells. J. Appl. Genet. 2005, 46, 171–177. [Google Scholar] [PubMed]
- Yu, G.M.; Tan, W. Melatonin inhibits lipopolysaccharide-induced inflammation and oxidative stress in cultured mouse mammary tissue. Mediat. Inflamm. 2019, 2019, 8597159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, G.X.; Tian, Y.G.; Wang, H.Y.; Liu, F.N.; Xie, G.H. Protective effects of melatonin on lipopolysaccharide-induced mastitis in mice. Int. Immunopharmacol. 2015, 29, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Bishayi, B.; Adhikary, R.; Nandi, A.; Sultana, S. Beneficial effects of exogenous melatonin in acute Staphylococcus aureus and Escherichia coli infection-induced inflammation and associated behavioral response in mice after exposure to short photoperiod. Inflammation 2016, 39, 2072–2093. [Google Scholar] [CrossRef]
- Masadeh, M.M.; Alzoubi, K.H.; Al-azzam, S.I.; Khabour, O.F.; Al-buhairan, A.M. Ciprofloxacin-induced antibacterial activity is attenuated by pretreatment with antioxidant agents. Pathogens 2016, 5, 28. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Sun, P. Insight of Melatonin: The Potential of Melatonin to Treat Bacteria-Induced Mastitis. Antioxidants 2022, 11, 1107. https://doi.org/10.3390/antiox11061107
Li H, Sun P. Insight of Melatonin: The Potential of Melatonin to Treat Bacteria-Induced Mastitis. Antioxidants. 2022; 11(6):1107. https://doi.org/10.3390/antiox11061107
Chicago/Turabian StyleLi, Hongyang, and Peng Sun. 2022. "Insight of Melatonin: The Potential of Melatonin to Treat Bacteria-Induced Mastitis" Antioxidants 11, no. 6: 1107. https://doi.org/10.3390/antiox11061107
APA StyleLi, H., & Sun, P. (2022). Insight of Melatonin: The Potential of Melatonin to Treat Bacteria-Induced Mastitis. Antioxidants, 11(6), 1107. https://doi.org/10.3390/antiox11061107