Bioavailability of Organosulfur Compounds after the Ingestion of Black Garlic by Healthy Humans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
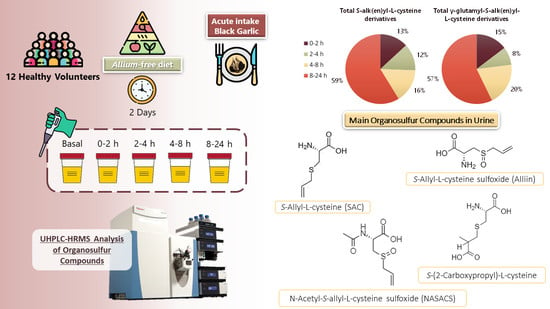
2.2. Study Design
2.3. Processing of Urine Samples
2.4. Chromatographic Analysis of Organosulfur Compounds in Urine Samples
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterisation of Organosulfur Compounds in Black Garlic
3.2. Identification and Quantification of Organosulfur Compounds and their Metabolites in Urine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amagase, H.; Petesch, B.L. Garlic. In Encyclopedia of Food Sciences and Nutrition; Academic Press: Cambridge, MA, USA, 2003; pp. 2861–2864. [Google Scholar]
- Bagul, M.; Kakumanu, S.; Wilson, T.A. Crude Garlic Extract Inhibits Cell Proliferation and Induces Cell Cycle Arrest and Apoptosis of Cancer Cells In Vitro. J. Med. Food 2015, 18, 731–737. [Google Scholar] [CrossRef]
- Shin, S.-S.; Song, J.-H.; Hwang, B.; Noh, D.-H.; Park, S.L.; Kim, W.T.; Park, S.-S.; Kim, W.-J.; Moon, S.-K. HSPA6 augments garlic extract-induced inhibition of proliferation, migration, and invasion of bladder cancer EJ cells; Implication for cell cycle dysregulation, signaling pathway alteration, and transcription factor-associated MMP-9 regulation. PLoS ONE 2017, 12, e0171860. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Feng, J.; Zhang, D.; Zhang, B.; Luo, M.; Su, D.; Lin, N. S-allylcysteine, a garlic derivative, suppresses proliferation and induces apoptosis in human ovarian cancer cells in vitro. Acta Pharmacol. Sin. 2013, 35, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Jang, E.K.; Seo, J.H.; Lee, S.P. Physiological activity and antioxidative effects of aged black garlic (Allium sativum L.) extract. Korean J. Food Sci. Technol. 2008, 40, 443–448. [Google Scholar]
- Kang, O.-J. Physicochemical Characteristics of Black Garlic after Different Thermal Processing Steps. Prev. Nutr. Food Sci. 2016, 21, 348. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Kang, O.J.; Gweon, O.C. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J. Funct. Foods 2013, 5, 80–86. [Google Scholar] [CrossRef]
- Ríos-Ríos, K.L.; Montilla, A.; Olano, A.; Villamiel, M. Physicochemical changes and sensorial properties during black garlic elaboration: A review. Trends Food Sci. Technol. 2019, 88, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Toledano Medina, M.A.; Pérez-Aparicio, J.; Moreno-Rojas, R.; Merinas-Amo, T. Evolution of some physicochemical and antioxidant properties of black garlic whole bulbs and peeled cloves. Food Chem. 2016, 199, 135–139. [Google Scholar] [CrossRef]
- Moreno-Ortega, A.; Pereira-Caro, G.; Ordóñez, J.L.; Moreno-Rojas, R.; Ortíz-Somovilla, V.; Moreno-Rojas, J.M. Bioaccessibility of Bioactive Compounds of ‘Fresh Garlic’ and ‘Black Garlic’ through In Vitro Gastrointestinal Digestion. Foods 2020, 9, 1582. [Google Scholar] [CrossRef]
- Ahmed, T.; Wang, C.-K. Black Garlic and Its Bioactive Compounds on Human Health Diseases: A Review. Molecules 2021, 26, 5028. [Google Scholar] [CrossRef]
- Gomez, C.D.; Aguilera, P.; Ortiz-Plata, A.; López, F.N.; Chánez-Cárdenas, M.E.; Flores-Alfaro, E.; Ruiz-Tachiquín, M.E.; Espinoza-Rojo, M. Aged garlic extract and S-allylcysteine increase the GLUT3 and GCLC expression levels in cerebral ischemia. Adv. Clin. Exp. Med. 2019, 28, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Alkreathy, H.M.; AlShehri, N.F.; Kamel, F.O.; Alghamdi, A.K.; Esmat, A.; Karim, S. Aged garlic extract potentiates doxorubicin cytotoxicity in human breast cancer cells. Trop. J. Pharm. Res. 2020, 19, 1669–1676. [Google Scholar] [CrossRef]
- Dong, M.; Yang, G.; Liu, H.; Liu, X.; Lin, S.; Sun, D.; Wang, Y. Aged black garlic extract inhibits Ht29 colon cancer cell growth via the PI3K/Akt signaling pathway. Biomed. Reports 2014, 2, 250–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, K.; Billington, D. Dietary Supplementation with Aged Garlic Extract Inhibits ADP-Induced Platelet Aggregation in Humans. J. Nutr. 2000, 130, 2662–2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, M.; Li, W. Aged Garlic Extract, a Modulator of Cardiovascular Risk Factors: A Dose-Finding Study on the Effects of AGE on Platelet Functions. J. Nutr. 2001, 131, 980S–984S. [Google Scholar] [CrossRef] [Green Version]
- Wlosinska, M.; Nilsson, A.C.; Hlebowicz, J.; Hauggaard, A.; Kjellin, M.; Fakhro, M.; Lindstedt, S. The effect of aged garlic extract on the atherosclerotic process—A randomized double-blind placebo-controlled trial. BMC Complement. Med. Ther. 2020, 20, 132. [Google Scholar] [CrossRef]
- Morihara, N.; Hino, A.; Yamaguchi, T.; Suzuki, J. Aged Garlic Extract Suppresses the Development of Atherosclerosis in Apolipoprotein E–Knockout Mice. J. Nutr. 2016, 146, 460S–463S. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.H.; Jeong, H.R.; Jo, Y.N.; Kim, H.J.; Shin, J.H.; Heo, H.J. Ameliorating effects of aged garlic extracts against Aβ-induced neurotoxicity and cognitive impairment. BMC Complement. Altern. Med. 2013, 13, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Shiju, T.; Rajesh, N.; Viswanathan, P. Renoprotective effect of aged garlic extract in streptozotocin-induced diabetic rats. Indian J. Pharmacol. 2013, 45, 23. [Google Scholar] [CrossRef]
- Chen, Y.A.; Tsai, J.C.; Cheng, K.C.; Liu, K.F.; Chang, C.K.; Hsieh, C.W. Extracts of black garlic exhibits gastrointestinal motility effect. Food Res. Int. 2018, 107, 102–109. [Google Scholar] [CrossRef]
- Moreno-Ortega, A.; Di Pede, G.; Pereira-Caro, G.; Calani, L.; Mena, P.; Del Rio, D.; Moreno-Rojas, J.M. In Vitro Colonic Fermentation of (Poly)phenols and Organosulfur Compounds of Fresh and Black Garlic. J. Agric. Food Chem. 2022, 70, 3666–3677. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Huber, K.; Popp, M.; Bauer, P.; Buettner, A.; Sharapa, C.; Scheffler, L.; Loos, H.M. Quantification of Allyl Methyl Sulfide, Allyl Methyl Sulfoxide, and Allyl Methyl Sulfone in Human Milk and Urine after Ingestion of Cooked and Roasted Garlic. Front. Nutr. 2020, 7, 565496. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.T.T.; Hiserodt, R.D.; Fukuda, E.K.; Ruiz, R.J.; Zhou, Z.; Lech, J.; Rosen, S.L.; Hartman, T.G. The determination of metabolites of garlic preparations in breath and human plasma. BioFactors 2000, 13, 241–249. [Google Scholar] [CrossRef]
- Scheffler, L.; Sharapa, C.; Buettner, A. Quantification of volatile metabolites derived from garlic (Allium sativum) in human urine. Front. Nutr. 2019, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, L.; Sharapa, C.; Buettner, A. Quantification of volatile metabolites derived from garlic in human breast milk. Food Chem. 2019, 274, 603–610. [Google Scholar] [CrossRef]
- Scheffler, L.; Sauermann, Y.; Heinlein, A.; Sharapa, C.; Buettner, A. Detection of Volatile Metabolites Derived from Garlic (Allium sativum) in Human Urine. Metabolites 2016, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Nagae, S.; Ushijima, M.; Hatono, S.; Imai, J.; Kasuga, S.; Matsuura, H.; Itakura, Y.; Higashi, Y. Pharmacokinetics of the garlic compound S-allylcysteine. Planta Med. 1994, 60, 214–217. [Google Scholar] [CrossRef]
- Ichikawa, M.; Mizuno, I.; Yoshida, J.; Ide, N.; Ushijima, M.; Kodera, Y.; Hayama, M.; Ono, K. Pharmacokinetics of cycloalliin, an organosulfur compound found in garlic and onion, in rats. J. Agric. Food Chem. 2006, 54, 9811–9819. [Google Scholar] [CrossRef]
- Moreno-Rojas, J.M.; Moreno-Ortega, A.; Ordóñez, J.L.; Moreno-Rojas, R.; Pérez-Aparicio, J.; Pereira-Caro, G. Development and validation of UHPLC-HRMS methodology for the determination of flavonoids, amino acids and organosulfur compounds in black onion, a novel derived product from fresh shallot onions (Allium cepa var. aggregatum). LWT 2018, 97, 376–383. [Google Scholar] [CrossRef]
- Kodera, Y.; Suzuki, A.; Imada, O.; Kasuga, S.; Sumioka, I.; Kanezawa, A.; Taru, N.; Fujikawa, M.; Nagae, S.; Masamoto, K.; et al. Physical, chemical, and biological properties of S-allylcysteine, an amino acid derived from garlic. J. Agric. Food Chem. 2002, 50, 622–632. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, M.J.; Wang, C.; Zhou, H.; Fan, L.; Huang, X. Thermolysis kinetics and thermal degradation compounds of alliin. Food Chem. 2017, 223, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, Y.; Sun, B.; Wang, C.; Mo, Y. Effect of ultrasound on the activity of alliinase from fresh garlic. Ultrason. Sonochem. 2011, 18, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S. Allicin and Other Functional Active Components in Garlic: Health Benefits and Bioavailability. Int. J. Food Prop. 2007, 10, 245–268. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Sanches-Silva, A.; Daglia, M.; Nabavi, S.F.; Jafari, N.J.; Izadi, M.; Ajami, M.; Nabavi, S.M. Antifungal and antibacterial activities of allicin: A review. Trends Food Sci. Technol. 2016, 52, 49–56. [Google Scholar] [CrossRef]
- Kodera, Y.; Ushijima, M.; Amano, H.; Suzuki, J.I.; Matsutomo, T. Chemical and Biological Properties of S-1-Propenyl-l-Cysteine in Aged Garlic Extract. Molecules 2017, 22, 570. [Google Scholar] [CrossRef] [Green Version]
- Kodera, Y.; Matsutomo, T.; Itoh, K. The Evidence for the Production Mechanism of cis-S-1-Propenylcysteine in Aged Garlic Extract Based on a Model Reaction Approach Using Its Isomers and Deuterated Solvents. Planta Medica Lett. 2015, 2, e69–e72. [Google Scholar] [CrossRef] [Green Version]
- Matsutomo, T.; Kodera, Y. Development of an Analytic Method for Sulfur Compounds in Aged Garlic Extract with the Use of a Postcolumn High Performance Liquid Chromatography Method with Sulfur-Specific Detection. J. Nutr. 2016, 146, 450S–455S. [Google Scholar] [CrossRef] [Green Version]
- Verhagen, H.; Hageman, G.J.; Rauma, A.-L.; Versluis-de Haan, G.; van Herwijnen, M.H.M.; de Groot, J.; Törrönen, R.; Mykkänen, H. Biomonitoring the intake of garlic via urinary excretion of allyl mercapturic acid. Br. J. Nutr. 2001, 86, S111–S114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amano, H.; Kazamori, D.; Itoh, K.; Kodera, Y. Metabolism, Excretion, and Pharmacokinetics of S-Allyl-l-Cysteine in Rats and Dogs. Drug Metab. Dispos. 2015, 43, 749–755. [Google Scholar] [CrossRef] [Green Version]
- Munck, L.K.; Munck, B.G. Amino Acid Transport in the Small Intestine. Physiol. Res. 1994, 43, 335–345. [Google Scholar] [CrossRef]
- Bröer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silbernagl, S.; Foulkes, E.C.; Deetjen, P. Renal transport of amino acids. Rev. Physiol. Biochem. Pharmacol. 1975, 74, 105–167. [Google Scholar] [PubMed]
- Krause, R.J.; Glocke, S.C.; Elfarra, A.A. Sulfoxides as Urinary Metabolites of S-Allyl-L-Cysteine in Rats: Evidence for the Involvement of Flavin-Containing Monooxygenases. Drug Metab. Dispos. 2002, 30, 1137–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cope, K.; Seifried, H.; Seifried, R.; Milner, J.; Kris-Etherton, P.; Harrison, E.H. A gas chromatography–mass spectrometry method for the quantitation of N-nitrosoproline and N-acetyl-S-allylcysteine in human urine: Application to a study of the effects of garlic consumption on nitrosation. Anal. Biochem. 2009, 394, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, Y.; Kumagai, H. Characteristics, biosynthesis, decomposition, metabolism and functions of the garlic odour precursor, S-allyl-l-cysteine sulfoxide (Review). Exp. Ther. Med. 2020, 19, 1528–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Praticò, G.; Gao, Q.; Manach, C.; Dragsted, L.O. Biomarkers of food intake for Allium vegetables. Genes Nutr. 2018, 13, 34. [Google Scholar] [CrossRef] [Green Version]
- Edmands, W.M.B.; Beckonert, O.P.; Stella, C.; Campbell, A.; Lake, B.G.; Lindon, J.C.; Holmes, E.; Gooderham, N.J. Identification of Human Urinary Biomarkers of Cruciferous Vegetable Consumption by Metabonomic Profiling. J. Proteome Res. 2011, 10, 4513–4521. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, Y.; Honma, R.; Yazaki, T.; Shibuya, T.; Sakaguchi, T.; Uto-Kondo, H.; Kumagai, H. Sulfuric Odor Precursor S-Allyl-l-Cysteine Sulfoxide in Garlic Induces Detoxifying Enzymes and Prevents Hepatic Injury. Antioxidants 2019, 8, 385. [Google Scholar] [CrossRef]
- Rojas, P.; Serrano-García, N.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Maldonado, P.D.; Ruiz-Sánchez, E. S-Allylcysteine, a garlic compound, protects against oxidative stress in 1-methyl-4-phenylpyridinium-induced parkinsonism in mice. J. Nutr. Biochem. 2011, 22, 937–944. [Google Scholar] [CrossRef]
- Ashafaq, M.; Khan, M.M.; Shadab Raza, S.; Ahmad, A.; Khuwaja, G.; Javed, H.; Khan, A.; Islam, F.; Siddiqui, M.S.; Safhi, M.M.; et al. S-allyl cysteine mitigates oxidative damage and improves neurologic deficit in a rat model of focal cerebral ischemia. Nutr. Res. 2012, 32, 133–143. [Google Scholar] [CrossRef]
- Komatsu, W.; Miura, Y.; Yagasaki, K. Suppression of hypercholesterolemia in hepatoma-bearing rats by cabbage extract and its component, S-methyl-l-cysteine sulfoxide. Lipids 1998, 33, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Marks, H.S.; Anderson, J.A.; Stoewsand, G.S. Effect of s-methyl cysteine sulphoxide and its metabolite methyl methane thiosulphinate, both occurring naturally in brassica vegetables, on mouse genotoxicity. Food Chem. Toxicol. 1993, 31, 491–495. [Google Scholar] [CrossRef] [PubMed]






| Black Garlic | µmol | % in Black Garlic |
|---|---|---|
| γ-Glutamyl-S-alk(en)yl-L-cysteine derivatives (GSAk) | ||
| γ–Glutamyl-S-propyl-L-cysteine sulfoxide (GSPCS) | 0.86 ± 0.07 | 0.2 |
| γ-Glutamyl-S-(2-carboxypropyl)-L-cysteine | 1.00 ± 0.07 | 0.2 |
| γ-Glutamyl-S-methyl-L-cysteine (GSMC) | 3.98 ± 0.00 | 0.8 |
| γ-Glutamyl-S-allyl-L-cysteine (GSAC) | 87.9 ± 0.4 | 17.2 |
| γ-Glutamyl-S-allyl-L-cysteine sulfoxide (GSACS) | 69.0 ± 0.3 | 13.5 |
| γ-Glutamyl-S-methyl-L-cysteine sulfoxide (GSMCS) | 7.60 ± 0.02 | 1.5 |
| γ–Glutamyl-S-propyl-L-cysteine (GSPC) | 0.90 ± 0.08 | 0.2 |
| γ-Glutamyl-S-(2-carboxyethyl)-L-cysteine-glycine | 2.38 ± 0.01 | 0.5 |
| γ-Glutamyl-S-allylthio-L-cysteine | 10.08 ± 0.02 | 2.0 |
| γ-Glutamyl-S-(2-carboxypropyl)-L-cysteine-glycine | 3.03 ± 0.21 | 0.6 |
| Total GSAk | 186.7 ± 0.7 | 36.6 |
| S-alk(en)yl-L-cysteine derivatives (SAk) | ||
| S-Allyl-L-cysteine (SAC) | 153.7 ± 0.3 | 30.1 |
| S-Allyl-L-cysteine sulfoxide (Alliin) | 112.2 ± 0.6 | 22 |
| S-(2-Carboxypropyl)-L-cysteine | 2.30 ± 0.00 | 0.5 |
| S-Methyl-L-cysteine sulfoxide (Methiin) | 4.86 ± 0.03 | 1.0 |
| Methionine sulfoxide | 10.04 ± 0.03 | 2.0 |
| S-(2-carboxypropyl)-L-cysteine-glycine | 5.20 ± 0.22 | 1.0 |
| S-Methyl-L-cysteine (Deoxymethiin) | 3.36 ± 0.02 | 0.7 |
| S-Allylmercapto-L-cysteine (SAMC) | 8.16 ± 0.02 | 1.6 |
| S-Propylmercapto-L-cysteine (SPMC) | 4.58 ± 0.01 | 0.9 |
| S-Allylsulfenic acid | 18.70 ± 0.05 | 3.7 |
| Total SAk | 323.1 ± 0.9 | 63.4 |
| Total Organosulfur Compounds | 509.8 ± 1.6 | 100 |
| Peak | Retention Time (min) | Compound | Chemical Formula [m/z]- | Experimental Mass [m/z]- | δ (ppm) |
|---|---|---|---|---|---|
| γ-Glutamyl-S-alk(en)yl-L-cysteine derivatives (GSAk) | |||||
| 1 | 4.34 | γ-Glutamyl-S-(propyl)-L-cysteine sulfoxide (GSPCS) | C11H21N2O6S | 309.1115 | −2.1191 |
| 2 | 6.34 | γ-Glutamyl-S-(S-1-propenyl)-L-cysteine-glycine | C13H22N3O6S2 | 380.0944 | 0.8362 |
| 3 | 6.43 | γ-Glutamyl-S-(2-carboxypropyl)-L-cysteine | C12H19N2O7S−2 | 335.0907 | −0.0579 |
| 4 | 7.86 | γ-Glutamyl-S-(S-methyl)-L-cysteine-glycine | C11H20N3O6S2 | 354.0788 | 0.6916 |
| 5 | 7.88 | γ-Glutamyl-S-methyl-L-cysteine (GSMC) | C9H17N2O5S | 265.0853 | 0.1777 |
| 6 | 8.53 | Glutathione | C10H18N3O6S | 308.0911 | 0.7967 |
| 7 | 8.55 | γ-Glutamyl-S-allyl-L-cysteine (GSAC) | C11H19N2O5S | 291.1009 | 0.4285 |
| 8 | 8.71 | γ-Glutamyl-L-cysteine | C8H15N2O5S | 251.0696 | −0.9444 |
| 9 | 8.75 | γ-Glutamyl-S-(1-propenyl)-L-cysteine sulfoxide (GS1PCS) | C11H19N2O6S | 307.0958 | −0.4247 |
| 10 | 9.00 | γ-Glutamyl-S-allyl-L-cysteine sulfoxide (GSACS) | C11H19N2O6S | 307.0958 | −1.0128 |
| 11 | 9.61 | γ-Glutamyl-S-methyl-L-cysteine sulfoxide (GSMCS) | C9H17N2O6S | 281.0801 | −0.8092 |
| 12 | 9.63 | γ-Glutamyl-S-(propyl)-L-cysteine (GSPC) | C11H21N2O5S | 293.1165 | −0.3883 |
| S-alk(en)yl-L-cysteine derivatives (SAk) | |||||
| 13 | 1.39 | S-(2-Propenyl)-allyl-L-cysteine sulfoxide | C9H16NO3S | 218.0845 | −1.8770 |
| 14 | 3.92 | N-Acetyl-S-allyl-L-cysteine sulfoxide (NASACS) | C8H14NO4S | 220.0638 | −1.2877 |
| 15 | 3.96 | S-Butanoyl-L-cysteine sulfoxide (Butiin) | C7H16NO3S | 194.0845 | −0.8589 |
| 16 | 4.00 | S-Allyl-L-cysteine (SAC) | C6H12NO2S | 162.0583 | −1.0605 |
| 17 | 4.21 | N-Acetyl-S-allyl-L-cysteine (NASAC) | C8H14NO3S | 204.0689 | −1.9576 |
| 18 | 4.97 | trans-S-(1-Propenyl)-L-cysteine (S1PC) | C6H12NO2S | 162.0583 | −0.5898 |
| 19 | 5.60 | S-Propyl-L-cysteine (Deoxypropiin) | C6H14NO2S | 164.0740 | −4.2089 |
| 20 | 6.18 | S-Allyl-L-cysteine sulfoxide (Alliin) | C6H12NO3S | 178.0532 | 1.5374 |
| 21 | 6.70 | S-(1-Propenyl)-L-cysteine sulfoxide (Isoalliin) | C6H12NO3S | 178.0532 | 2.5793 |
| 22 | 7.10 | Cycloalliin | C6H12NO3S | 178.0532 | 2.3222 |
| 23 | 7.13 | S-(2-Carboxypropyl)-L-cysteine | C7H14NO4S | 208.0638 | 1.7404 |
| 24 | 7.26 | S-Methyl-L-cysteine sulfoxide (Methiin) | C4H10NO3S | 152.0376 | 3.5869 |
| 25 | 7.56 | Methionine sulfoxide | C5H12NO3S | 166.0532 | −0.6264 |
| 26 | 7.87 | S-Propyl-L-cysteine sulfoxide (Propiin) | C6H14NO3S | 180.0689 | 2.4960 |
| 27 | 8.09 | S-Carboxymethyl-L-cysteine (Carbocysteine) | C5H10NO4S | 180.0325 | 2.5441 |
| 28 | 8.43 | S-(2-Carboxypropyl)-L-cysteine-glycine | C9H17N2O5S | 265.0853 | 0.6382 |
| 29 | 8.55 | S-Ethyl-L-cysteine sulfoxide (Ethiin) | C5H12NO3S | 166.0532 | −0.0752 |
| 30 | 9.50 | Ajoene | C9H15OS3 | 235.0280 | −1.1224 |
| 31 | 9.58 | N-Acetyl-S-(2-carboxypropyl)-L-cysteine (NACPC) | C9H16NO5S | 250.0744 | 2.5686 |
| 32 | 9.74 | S-Allylmercaptoglutathione | C13H22N3O6S2 | 380.0945 | 0.2443 |
| 33 | 10.89 | Allixin | C12H19O4 | 227.1278 | −1.6378 |
| Peak | Compounds (nmol) | 0–2 h | 2–4 h | 4–8 h | 8–24 h | 0–24 h | % Excretion |
|---|---|---|---|---|---|---|---|
| γ-Glutamyl-S-alk(en)yl-L-cysteine derivatives (GSAk) | |||||||
| 1 | γ-Glutamyl-S-(propyl)-L-cysteine sulfoxide | 99 ± 0 a | 48 ± 12 a | 87 ± 19 a | 88 ± 28 a | 323 ± 59 | 37.6 |
| 2 | γ-Glutamyl-S-(S-1-propenyl)-L-cysteine-glycine | 1.6 ± 0.2 c | 1.0 ± 0.0 bc | 6.9 ± 0.0 a | 2.5 ± 0.0 b | 12.1 ± 0.2 | - |
| 3 | γ-Glutamyl-S-(2-carboxypropyl)-L-cysteine | 15 ± 3 a | 10 ± 3 a | 14 ± 2 a | 27 ± 11 a | 66 ± 19 | 6.6 |
| 4 | γ-Glutamyl-S-(S-methyl)-L-cysteine-glycine | 29 ± 4 b | 22 ± 4 b | 47 ± 7 b | 157 ± 25 a | 255 ± 40 | - |
| 5 | γ-Glutamyl-S-methyl-L-cysteine (GSMC) | 60 ± 11 b | 37 ± 4 b | 101 ± 24 b | 371 ± 65 a | 568 ± 104 | 14.3 |
| 6 | Glutathione | 21 ± 3 b | 10 ± 2 b | 31 ± 5 ab | 60 ± 13 a | 122 ± 22 | - |
| 7 | γ-Glutamyl-S-allyl-L-cysteine (GSAC) | 19 ± 5 b | 11 ± 2 b | 17 ± 4 b | 42 ± 9 a | 89 ± 20 | 0.1 |
| 8 | γ-Glutamyl-L-cysteine | 37 ± 8 b | 18 ± 3 b | 41 ± 11 ab | 89 ± 18 a | 185 ± 40 | - |
| 9 | γ-Glutamyl S-(1-propenyl)-L-cysteine sulfoxide (GS1PCS) | 3.1 ± 0.4 a | 1.1 ± 0.3 a | 1.7 ± 0.2 a | 3.2 ± 0.1 a | 9.1 ± 1.0 | - |
| 10 | γ-Glutamyl-S-allyl-L-cysteine sulfoxide (GSACS) | 13 ± 3 ab | 8 ± 1 b | 13 ± 3 ab | 24 ± 4 a | 58 ± 11 | 0.08 |
| 11 | γ-Glutamyl-S-methyl-L-cysteine sulfoxide (GSMCS) | 43 ± 12 b | 29 ± 9 b | 69 ± 25 b | 166 ± 35 a | 308 ± 82 | 4.05 |
| 12 | γ-Glutamyl-S-(propyl)-L-cysteine | 17 ± 3 b | 8 ± 1 b | 14 ± 4 b | 37 ± 7 a | 76 ± 15 | 8.4 |
| Total GSAk | 251 ± 41 b | 144 ± 21 b | 339 ± 77 b | 980 ± 134 a | 1713 ± 273 | 0.92 | |
| S-alk(en)yl-L-cysteine derivatives (SAk) | |||||||
| 13 | S-(2-Propenyl)-allyl-L-cysteine sulfoxide | 179 ± 37 a | 98 ± 23 a | 162 ± 90 a | 238 ± 69 a | 678 ± 220 | - |
| 14 | N-acetyl-S-allyl-L-cysteine sulfoxide (NASACS) | 144 ± 35 a | 118 ± 45 a | 144 ± 40 a | 672 ± 391 a | 1079 ± 512 | - |
| 15 | S-Butanoyl-L-cysteine sulfoxide (Butiin) | 17 ± 3 a | 34 ± 16 a | 14 ± 3 a | 94 ± 28 a | 159 ± 50 | - |
| 16 | S-Allyl-L-cysteine (SAC) | 302 ± 64 b | 348 ± 112 b | 391 ± 73 ab | 1950 ± 810 a | 2991 ± 1059 | 1.95 |
| 17 | N-acetyl-S-allyl-L-cysteine (NASAC) | 1.49 ± 0.00 a | 10 ± 2 a | 3.4 ± 0.1 a | 8.6 ± 1.7 a | 23 ± 3 | - |
| 18 | tranS-S-(1-Propenyl)-L-cysteine (S1PC) | 618 ± 27 a | 982 ± 151 a | 448 ± 78 a | 1862 ± 731 a | 3911 ± 987 | - |
| 19 | S-Propyl-L-cysteine (Deoxypropiin) | 1417 ± 201 b | 1005 ± 106 b | 1387 ± 406 b | 3226 ± 679 a | 7035 ± 1392 | - |
| 20 | S-Allyl-L-cysteine sulfoxide (Alliin) | 97 ± 15 a | 144 ± 35 a | 105 ± 25 a | 1037 ± 961 a | 1383 ± 1035 | 1.23 |
| 21 | traN-S-(1-Propenyl)-L-cysteine sulfoxide (Isoalliin) | 1825 ± 629 a | 2483 ± 704 a | 2059 ± 634 a | 8633 ± 7274 a | 15,001 ± 9241 | - |
| 22 | Cycloalliin | 120 ± 18 ab | 244 ± 42 a | 198 ± 47 a | 63 ± 15 b | 625 ± 122 | - |
| 23 | S-(2-Carboxypropyl)-L-cysteine | 382 ± 59 a | 275 ± 45 a | 389 ± 78 a | 7759 ± 7038 a | 8804 ± 7220 | 382.8 |
| 24 | S-Methyl-L-cysteine sulfoxide (Methiin) | 2854 ± 763 | 2203 ± 518 | 3596 ± 1205 | 9300 ± 3554 | 17,954 ± 6040 | 369.4 |
| 25 | Methionine sulfoxide | 237 ± 29 b | 387 ± 61 b | 567 ± 94 b | 1029 ± 159 a | 2221 ± 343 | 22.1 |
| 26 | S-Propyl-L-cysteine sulfoxide (Propiin) | 165 ± 35 b | 131 ± 21 b | 196 ± 26 b | 533 ± 106 a | 1024 ± 189 | - |
| 27 | S-Carboxymethyl-L-cysteine (Carbocysteine) | 59 ± 8 b | 61 ± 9 b | 94 ± 19 b | 222 ± 41 a | 435 ± 78 | - |
| 28 | S-(2-Carboxypropyl)-L-cysteine-glycine | 232 ± 79 ab | 89 ± 22 b | 248 ± 77 ab | 472 ± 85 a | 1041 ± 264 | 20.0 |
| 29 | S-Ethyl-L-cysteine sulfoxide (Ethiin) | 65 ± 16 b | 42 ± 8 b | 98 ± 27 b | 229 ± 35 a | 435 ± 86 | - |
| 30 | Ajoene | 6.6 ± 1.4 b | 5.3 ± 0.8 b | 12 ± 2 b | 31 ± 7 a | 55 ± 11 | - |
| 31 | N-acetyl-S-(2-carboxypropyl)-L-cysteine (NACPC) | 37 ± 5 b | 19 ± 2 b | 57 ± 8 ab | 80 ± 11 a | 193 ± 27 | - |
| 32 | S-Allylmercaptoglutathione | 1.8 ± 0.4 b | <LOD | 1.5 ± 0.1 b | 5.0 ± 0.3 a | 8.3 ± 0.8 | - |
| 33 | Allixin | <LOD | 13 ± 4 a | 19 ± 1 a | 9.5 ± 2.a | 41 ± 8 | - |
| Total SAk | 8200 ± 1605 | 7810 ± 1506 | 9802 ± 2619 | 36,787 ± 20,673 | 62,599 ± 26,403 | 19.4 | |
| Total Organosulfur Compounds | 8451 ± 1630 | 7953 ± 1518 | 10,141 ± 2690 | 37,767 ± 20,746 | 64,312 ± 26,584 | 12.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Ortega, A.; Pereira-Caro, G.; Ludwig, I.A.; Motilva, M.-J.; Moreno-Rojas, J.M. Bioavailability of Organosulfur Compounds after the Ingestion of Black Garlic by Healthy Humans. Antioxidants 2023, 12, 925. https://doi.org/10.3390/antiox12040925
Moreno-Ortega A, Pereira-Caro G, Ludwig IA, Motilva M-J, Moreno-Rojas JM. Bioavailability of Organosulfur Compounds after the Ingestion of Black Garlic by Healthy Humans. Antioxidants. 2023; 12(4):925. https://doi.org/10.3390/antiox12040925
Chicago/Turabian StyleMoreno-Ortega, Alicia, Gema Pereira-Caro, Iziar A. Ludwig, María-José Motilva, and José Manuel Moreno-Rojas. 2023. "Bioavailability of Organosulfur Compounds after the Ingestion of Black Garlic by Healthy Humans" Antioxidants 12, no. 4: 925. https://doi.org/10.3390/antiox12040925
APA StyleMoreno-Ortega, A., Pereira-Caro, G., Ludwig, I. A., Motilva, M. -J., & Moreno-Rojas, J. M. (2023). Bioavailability of Organosulfur Compounds after the Ingestion of Black Garlic by Healthy Humans. Antioxidants, 12(4), 925. https://doi.org/10.3390/antiox12040925