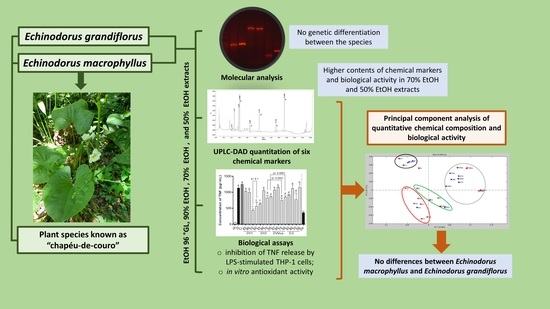
Quantitative Chemical Composition, Anti-Oxidant Activity, and Inhibition of TNF Release by THP-1 Cells Induced by Extracts of Echinodorus macrophyllus and Echinodorus grandiflorus
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Drug Samples
2.2. Chemicals and Reagents
2.3. DNA Isolation, Amplification, Sequencing, Alignment, and Pairwise Distance Calculation
2.4. Molecular Markers, Taxon Sampling, and Molecular Phylogenetic Analyses
2.5. Preparation of Extracts
2.6. Quantification of Chemical Markers by UPLC-DAD
2.6.1. Sample Preparation and Chromatographic and Analytical Conditions
2.6.2. Identification of Chemical Markers by LC-MS Analysis
2.6.3. Validation of the UPLC-DAD Method
2.7. Effect on the Release of TNF In Vitro
ELISA Protocols for In Vitro Assays
2.8. In Vitro Anti-Oxidant Activity
2.8.1. DPPH Radical Scavenger Activity
2.8.2. β-Carotene/Linoleic Acid Co-Oxidation Assay
2.8.3. ROS Activity in THP-1 Cells
2.9. Statistical Analysis
2.10. PCA Model
3. Results and Discussion
3.1. Molecular and Phylogenetic Analysis of E. macrophyllus
3.2. Development and Validation of an UPLC-DAD Method for the Analysis of Chemical Markers
3.3. Quantitation of Chemical Markers in Extracts of E. macrophyllus and E. grandiflorus
3.4. In Vitro Inhibition of TNF Release
3.5. In Vitro Anti-Oxidant Activity and Effect on ROS Production
3.6. PCA Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corrêa, M.P. Dictionary of Useful Plants in Brazil and Cultivated Exotics; National Press: Rio de Janeiro, Brazil, 1984. [Google Scholar]
- Lorenzi, H.; Matos, F.J.A. Plantas Medicinais No Brasil: Nativas e Exóticas; Instituto Plantarum: Nova Odessa, Brazil, 2000; p. 43. [Google Scholar]
- Tabosa, A.B.; Matias, L.Q.; Martins, F.R. Live fast and die young: The aquatic macrophyte dynamics in a temporary pool in the Brazilian semiarid region. Aquat. Bot. 2012, 102, 71–78. [Google Scholar] [CrossRef]
- Lorenzi, H. Weeds of Brazil: Terrestrial, Aquatic, Parasitic and Toxic, 6th ed.; Nova Odessa’s Edition; Editora Vozes: Petrópolis, Brazil, 1998; pp. 110–111. [Google Scholar]
- Brazil Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Farmacopeia Brasileira, 1st ed.; Agência Nacional de Vigilância Sanitária: São Paulo, Brazil, 1926; 1149p. [Google Scholar]
- Brazil Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Farmacopeia Brasileira, 2nd ed.; Agência Nacional de Vigilância Sanitária: São Paulo, Brazil, 1959; 208p. [Google Scholar]
- Brazil Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Farmacopeia Brasileira, 5th ed.; Agência Nacional de Vigilância Sanitária: São Paulo, Brazil, 2010; Volume 2, 808p, Available online: https://www2.fcfar.unesp.br/Home/Instituicao/Departamentos/principiosativosnaturaisetoxicologianovo/farmacognosia/5-edicao---volume-2.pdf (accessed on 14 April 2023).
- Brazil Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Farmacopeia Brasileira, 6th ed.; Agência Nacional de Vigilância Sanitária: São Paulo, Brazil, 2019; Volume 2, 746p. Available online: https://www.gov.br/anvisa/pt-br/assuntos/farmacopeia/farmacopeia-brasileira/6a-edicao-volume-2 (accessed on 14 April 2023).
- Marques, A.M.; Provance, D.W.; Kaplan, M.A.C.; Figueiredo, M.R. Echinodorus grandiflorus: Ethnobotanical, phytochemical and pharmacological overview of a medicinal plant used in Brazil. Food Chem. Toxicol. 2017, 109, 1032–1047. [Google Scholar] [CrossRef] [PubMed]
- Lehtone, S.L.; Myllys, L. Cladistic analysis of Echinodorus (Alismataceae): Simultaneous analysis of molecular and morphological data. Cladistics 2008, 24, 218–239. [Google Scholar] [CrossRef]
- Silva, G.P.; Fernandes, D.C.; Vigliano, M.V.; Pinto, F.A.; Fonseca, E.N.; Santos, S.V.M.; Marques, P.R.; Gayer, C.R.M.; Velozo, L.S.M.; Lima, C.K.F.; et al. Echinodorus macrophyllus: Hydroxycinnamoyl derivatives reduce neutrophil migration through modulation of cytokines, chemokines, and prostaglandin in the air-pouch model. J. Ethnopharmacol. 2022, 284, 114757. [Google Scholar] [CrossRef]
- Garcia, E.F.; Oliveira, M.A.; Dourado, L.P.A.; Souza, D.G.; Teixeira, M.M.; Braga, F.C. In vitro TNF-α inhibition elicited by extracts from Echinodorus grandiflorus leaves and correlation with their phytochemical composition. Planta Med. 2016, 82, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Garcia, E.; Oliveira, M.A.; Candido, L.C.M.; Coelho, F.M.; Costa, V.V.; Queiroz-Junior, C.M.; Boff, D.; Amaral, F.A.; Souza, D.G.; Teixeira, M.M.; et al. Effect of the hydroethanolic extract from Echinodorus grandiflorus leaves and a fraction enriched in flavone-C-glycosides on antigen-induced arthritis in mice. Planta Med. 2016, 82, 407–413. [Google Scholar] [CrossRef]
- Kobayashi, J.; Sekiguchi, M.; Shigemori, H.; Ohsaki, A.; Chapecoderins, A.-C. New labdane derived diterpenoids from Echinodorus macrophyllus. J. Nat. Prod. 2000, 63, 375–377. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.B.D.; Gomes, J.H.S.; Pereira, A.C.; de Pádua, R.M.; Côrtes, S.F.; Sena, M.M.; Braga, F.C. Definition of chemical markers for Hancornia speciosa Gomes by chemometric analysis based on the chemical composition of extracts, their vasorelaxant effect and α-glucosidase inhibition. J. Ethnopharmacol. 2022, 299, 115692. [Google Scholar] [CrossRef]
- Doyle, J.L.; Doyle, D.J. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Lehtonen, S.; Falck, D. Watery varieties: Aquarium plant diversity from aesthetic, commercial, and systematic perspectives. In Ornamental Plants: Types, Cultivation and Nutrition; Joshua, C.A., Ed.; Nova Science Publishers: New York, NY, USA, 2011; ISBN 978-1-61761-736-2. [Google Scholar]
- Staden, R.; Beal, K.F.; Bonfield, J.K. The Staden Package, 1998. Methods Mol. Biol. 2000, 132, 115–130. [Google Scholar] [CrossRef]
- Edgar, R.; Muscle, C. Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelchner, S.A. The Evolution of Non-Coding Chloroplast DNA and Its Application in Plant Systematics. Ann. Mo. Bot. Gard. 2000, 87, 482–498. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W. GLOVAP Nomenclature Part 1. The Global Flora: A Practical Flora to Vascular Plant Species of the World; Special Edition; Plant Gateway: Bradford, UK, 2018; Volume 4, pp. 1–155. [Google Scholar]
- Swofford, D.L. PAUP (Phylogenetic Analysis Using PAUP); Sinauer Associates: Sunderland, MA, USA, 2018; p. 4.0a159. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Kress, W.J.; Prince, L.M.; Williams, K.J. The phylogeny and a new classification of the gingers (Zingiberaceae): Evidence from molecular data. Am. J. Bot. 2002, 89, 1682–1696. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MRBAYES 3.2: Efficient Bayesian phylogenetic inference and model selection across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), Gateway Computing, New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Nylander, J.A.A. MrModeltest v2. Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Erixon, P.; Svennblad, B.; Britton, T.; Oxelman, B. Reliability of Bayesian posterior probabilities and bootstrap frequencies in phylogenetics. Syst. Biol. 2003, 52, 665–673. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Guidance for Industry: Analytical Procedures and Methods Validation. 2015. Available online: https://www.fda.gov/files/drugs/published/Analytical-Procedures-and-Methods-Validation-for-Drugs-and-Biologics.pdf (accessed on 13 March 2023).
- ICH—International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceutical for Human Use. ICH Harmonised Tripartite Guideline. In Validation of Analytical Procedures: Methodology, Q2B (EMA/CHMP/ICH/82072/2006); European Agency for the Evaluation of Medicinal Products: London, UK, 2006. [Google Scholar]
- Agência Nacional de Vigilância Sanitária—Brazil. RDC n° 166 de 24 of July of 2017. Provides for the Validation of Analytical Methods and Other Measures. Available online: http://antigo.anvisa.gov.br/documents/10181/2721567/RDC_166_2017_COMP.pdf/d5fb92b3-6c6b-4130-8670-4e3263763401#:~:text=QU%C3%8DMICAS%20DE%20REFER%C3%8ANCIA-,Art.,comp%C3%AAndios%20oficialmente%20reconhecidos%20pela%20Anvisa (accessed on 13 March 2023).
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; Dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Almeida, J.M.; dos Santos, R.J.; Genovese, M.I.; Lajolo, F.M. Avaliação da atividade antioxidante utilizando sistema beta-caroteno/ácido linoléico e método de sequestro de radicais DPPH•. Food Sci. Technol. 2006, 26, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Ryan, K.A.; Smith, M.F., Jr.; Sanders, M.K.; Ernst, P.B. Reactive oxygen and nitrogen species differentially regulate Toll-like receptor 4-mediated activation of NF-kappa B and interleukin-8 expression. Infect. Immun. 2004, 72, 2123–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; Mcginnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 1, 36. [Google Scholar] [CrossRef] [PubMed]
- CBOL Plant Working Group. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govaerts, R. Echinodorus. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet. Available online: http://www.plantsoftheworldonline.org/ (accessed on 26 March 2023).
- Schnitzler, M.; Petereit, F.; Nahrstedt, A. Trans-Aconitic acid, glucosylflavones and hydroxycinnamoyltartaric acids from the leaves of Echinodorus grandiflorus ssp. aureus, a Brazilian medicinal plant. Rev. Bras. Farmacogn. 2007, 17, 149–154. [Google Scholar] [CrossRef]
- Portella, V.G.; Cosenza, G.P.; Diniz, L.R.; Pacheco, L.F.; Cassali, G.D.; Caliari, M.V.; Brandão, M.D.; Vieira, M.A. Nephroprotective Effect of Echinodorus macrophyllus Micheli on Gentamicin-Induced Nephrotoxicity in Rats. Nephron Extra 2012, 2, 177–183. [Google Scholar] [CrossRef]
- Fernandes, D.C.; Martins, B.P.; Silva, G.P.D.; Fonseca, E.N.D.; Santos, S.V.M.; Velozo, L.S.M.; Gayer, C.R.M.; Sabino, K.C.C.; Coelho, M.G.P. Echinodorus macrophyllus fraction with a high level of flavonoid inhibits peripheral and central mechanisms of nociception. J. Tradit. Complement. Med. 2021, 12, 123–130. [Google Scholar] [CrossRef]
- Snyder, L.R.; Kirkland, J.J.; Glajch, J.L. Practical HPLC Method Development, 2nd ed.; Wiley-Interscience: New York, NY, USA, 1997; 765p. [Google Scholar]
- Strada, C.L.; Da Lima, K.C.; Da Silva, V.C.; Ribeiro, R.V.; Dores, E.F.G.C.; Dall’oglio, E.; Schmeda-Hirschmann, G.; Carollo, C.C.; Martins, D.T.O.; Junior, P.T.S. Isovitexin as marker and bioactive compound in the antinociceptive activity of the Brazilian crude drug extracts of Echinodorus scaber and E. grandiflorus. Rev. Bras. Farmacogn. 2017, 27, 619–626. [Google Scholar] [CrossRef]
- Rani, R.; Arora, S.; Kaur, J.; Manhas, R.K. Phenolic compounds as antioxidants and chemopreventive drugs from Streptomyces cellulosae strain TES17 isolated from rhizosphere of Camellia sinensis. BMC Complement. Altern. Med. 2018, 18, 82. [Google Scholar] [CrossRef] [Green Version]
- Gasparotto, F.M.; Palozi, R.A.C.; Da Silva, C.H.F.; Pauli, K.B.; Donadel, G.; Lourenço, B.H.L.B.; Nunes, B.C.; Lívero, F.A.D.R.; De Souza, L.M.; Lourenço, E.L.B.; et al. Antiatherosclerotic Properties of Echinodorus grandiflorus (Cham. & Schltdl.) Micheli: From Antioxidant and Lipid-Lowering Effects to an Anti-Inflammatory Role. J. Med. Food 2019, 22, 919–927. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 338. [Google Scholar] [CrossRef]
- Conforti, F.; Menichini, F. Phenolic compounds from plants as nitric oxide production inhibitors. Curr. Med. Chem. 2011, 18, 1137–1145. [Google Scholar] [CrossRef]
- Zielinska-Blizniewska, H.; Sitarek, P.; Merecz-Sadowska, A.; Malinowska, K.; Zajdel, K.; Jablonska, M.; Zajdel, R. Plant Extracts and Reactive Oxygen Species as Two Counteracting Agents with Anti- and Pro-Obesity Properties. Int. J. Mol. Sci. 2019, 20, 4556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxidative Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef] [Green Version]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [Green Version]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free. Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Prieto, M.A.; Rodríguez-Amado, I.; Vázquez, J.A.; Murado, M.A. β-Carotene assay revisited. application to characterize and quantify antioxidant and prooxidant activities in a microplate. J. Agric. Food Chem. 2012, 60, 8983–8993. [Google Scholar] [CrossRef] [Green Version]
- Bibi-Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety-Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Koleva, I.I.; Van Beek, T.A.; Linssen, J.P.; De Groot, A.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, B.; Alves, L.F.; Favoreto, R.F. Echinodorus grandiflorus, E. macrophyllus. In Monografias de Plantas Medicinais Brasileiras e Aclimatadas: Volume II [Online]; Abifisa; Editora FIOCRUZ: Rio de Janeiro, Brazil, 2022; pp. 97–120. ISBN 978-65-5708-177-8. [Google Scholar] [CrossRef]
- Pang, Z.; Chen, J.; Wang, T.; Gao, C.; Li, Z.; Guo, L.; Xu, J.; Cheng, Y. Linking Plant Secondary Metabolites and Plant Microbiomes: A Review. Front. Plant Sci. 2021, 2, 12. [Google Scholar] [CrossRef]
- Cox, A.V.; Bennet, M.D.; Dyer, T.A. Use of the polymerase chain reaction to detect spacer size heterogeneity in plant 5S-rRNA gene clusters and to locate such clusters in wheat (Triticum aestivum L.). Theor. Appl. Genet. 1992, 83, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Grob, G.B.J.; Gravendeel, B.; Eurlings, M.C.M. Potential phylogenetic utility of the nuclear FLORICAULA ⁄LEAFY second intron: Comparison with three cloroplast DNA regions in Amorphophallus (Araceae). Mol. Phylogenet. Evol. 2004, 30, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Hilu, K.W. Application of matK gene sequences to grass systematics. Can. J. Bot. 1996, 74, 125–134. [Google Scholar] [CrossRef]
- Sang, T.; Crawford, D.J.; Stuessy, T.F. Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae). Am. J. Bot. 1997, 84, 1120–1136. [Google Scholar] [CrossRef] [Green Version]
- Tate, J.A.; Simpson, B.B. Paraphyly of Tarasa (Malvaceae) and diverse origins of the polyploid species. Syst. Bot. 2003, 28, 723–737. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfund, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–332. [Google Scholar]






| Marker | Best Matches | % Identity | Identities bp | Query Cover (%) | Accession No. |
|---|---|---|---|---|---|
| ITS | Echinodorus osiris | 99.56 | 684/687 | 100 | KT437651 |
| Echinodorus longiscapus | 99.42 | 683/687 | 100 | KT437653 | |
| Echinodorus portoalegrensis | 99.42 | 683/687 | 100 | KT437648 | |
| Echinodorus barthii | 99.27 | 682/687 | 100 | KT437652 | |
| Echinodorus opacus | 99.13 | 681/687 | 100 | KT437650 | |
| psbA-trnH | Echinodorus paniculatus | 100.00 | 463/463 | 100 | HM367285 |
| Echinodorus grandiflorus | 99.78 | 462/463 | 100 | HM367294 | |
| Echinodorus longiscapus | 99.78 | 462/463 | 100 | HM367292 | |
| Echinodorus muricatus | 99.57 | 462/464 | 100 | DQ786514 | |
| Echinodorus cordifolius | 99.35 | 460/463 | 100 | HM367302 | |
| matK-trnK | Echinodorus paniculatus | 99.85 | 1309/1311 | 100 | OK587808 |
| Echinodorus cordifolius | 99.85 | 1309/1311 | 100 | OK587807 | |
| Echinodorus paniculatus | 99.85 | 1309/1311 | 100 | EF088097 | |
| Echinodorus longiscapus | 99.77 | 1308/1311 | 100 | EF088112 | |
| Echinodorus grandiflorus | 99.69 | 1307/1311 | 100 | EF088113 | |
| Leafy | Echinodorus cordifolius | 99.07 | 212/214 | 100 | HM367210 |
| Echinodorus paniculatus | 99.07 | 212/214 | 100 | EF088144 | |
| Echinodorus uruguayensis | 98.60 | 211/214 | 100 | EF088159 | |
| Echinodorus cordifolius | 98.60 | 211/214 | 100 | EF088172 | |
| Echinodorus bracteatus | 98.60 | 212/215 | 100 | EF088170 |
| Marker | Terminals | Characters | VI (%) | CI | RI | Model (hLRTs) |
|---|---|---|---|---|---|---|
| ITS | 37 | 725 | 100 (13.8%) | 0.71 | 0.8 | GTR+G |
| 5S-NTS | 28 | 266 | 98 (36.8%) | 0.68 | 0.7 | HKY+G |
| LEAFY | 43 | 306 | 33 (10.8%) | 0.93 | 0.95 | K80+G |
| matK-trnK | 35 | 1311 | 164 (12.5%) | 0.86 | 0.92 | GTR+G |
| psbA-trnH | 46 | 574 | 108 (18.8%) | 0.81 | 0.91 | GTR+G |
| Combined | 47 | 3182 | 503 (15.8%) | 0.73 | 0.82 |
| N | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Clade F | 30 | 0.007 | |||||||
| 2. E. glaucus | 1 | 0.029 | n/c | ||||||
| 3. Clade E | 3 | 0.051 | 0.052 | 0.026 | |||||
| 4. E. bracteatus | 1 | 0.042 | 0.050 | 0.046 | n/c | ||||
| 5. Clade D | 2 | 0.071 | 0.073 | 0.073 | 0.068 | 0.001 | |||
| 6. Clade B | 6 | 0.065 | 0.067 | 0.065 | 0.059 | 0.078 | 0.022 | ||
| 7. Clade A | 3 | 0.094 | 0.089 | 0.090 | 0.090 | 0.095 | 0.092 | 0.037 | |
| 8. E. berteroi | 1 | 0.109 | 0.112 | 0.109 | 0.107 | 0.121 | 0.111 | 0.134 | n/c |
| ITS | 5S-NTS | LEAFY | matK-trnK | psbA-trnH | Combined | |
|---|---|---|---|---|---|---|
| Clade F | 0.008 | 0.062 | 0.015 | 0.003 | 0.004 | 0.007 |
| Clade E | 0.033 | 0.067 | 0.047 | 0.012 | 0.025 | 0.026 |
| Clade D | 0.003 | 0.004 | 0.000 | 0.000 | 0.000 | 0.001 |
| Clade B | 0.043 | 0.167 | 0.019 | 0.008 | 0.015 | 0.022 |
| Clade A | 0.019 | 0.127 | 0.029 | 0.025 | 0.058 | 0.037 |
| Compound | Retention Factor (k) | USP Tailing Factor | Number of Theoretical Plates (N) | RSD Values of Retention Time (%) | Resolution (Rs) |
|---|---|---|---|---|---|
| cis- and trans-aconitic acid (1) | 0.49 | 1.07 | 5656 | 0.12 | 1.87 |
| homoorientin (2) | 4.49 | 1.29 | 7480 | 0.07 | 3.41 |
| swertisin (3) | 7.20 | 1.02 | 6441 | 0.40 | 1.50 |
| chicoric acid (4) | 9.40 | 1.19 | 6756 | 0.08 | 1.55 |
| caffeoyl-feruloyl-tartaric acid (5) | 13.38 | 1.13 | 2147 | 0.22 | 2.65 |
| di-feruloyl-tartaric acid (6) | 15.42 | 1.05 | 6857 | 0.11 | 2.00 |
| Compound | Regression Equation | Linear Range (μg) | r2 | LOQ (μg) | LOD (μg) | Intra-Day RSD | Inter-Day RSD | Recovery (% ± SD) |
|---|---|---|---|---|---|---|---|---|
| 1 | y = 43,603x − 19,041 | 6.25–100 | 0.9999 | 0.990 | 0.300 | 2.65 | 2.60 | 96.0 ± 3.9 110.8 ± 4.8 100.5 ± 3.8 |
| 2 | y = 38,042x − 14,758 | 1.25–20 | 0.9998 | 0.216 | 0.065 | 0.23 | 1.55 | 110.2 ± 0.8 95.8 ± 4.4 98.8 ± 2.1 |
| 3 | y = 9441x + 23,371 | 3.0–150 | 0.9969 | 1.928 | 0.584 | 4.90 | 4.27 | 99.5 ± 1.9 107.9 ± 1.1 100.4 ± 2.9 |
| 4 | y = 47,326x − 53,912 | 9.37–300 | 0.9993 | 0.048 | 0.014 | 0.84 | 1.79 |
99.9 ± 0.7 100.0 ± 1.1 98.9 ± 2.1 |
| Concentration (% w/w) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Plant Material | Extract | Extractive Yield (% w/w) | cis- and trans- Aconitic Acid (1) | Homoorientin (2) | Swertisin (3) | Chicoric Acid (4) | Caffeoyl-Feruloyl-Tartaric Acid (5) | Di-Feruloyl-Tartaric Acid (6) |
| DV1 | 96 °GL EtOH | 8.51 | 0.491 ± 0.012 a | 0.052 ± 0.001 a | 0.171 ± 0.002 a | 0.750 ± 0.013 a | 0.636 ± 0.003 a | 0.630 ± 0.005 a |
| 90% EtOH | 9.98 | 2.992 ± 0.036 b | 0.095 ± 0.002 b | 0.260 ± 0.013 b | 1.531 ± 0.012 b | 0.794 ± 0.002 b | 0.751 ± 0.006 b | |
| 70% EtOH | 21.31 | 8.094 ± 0.024 c | 0.164 ± 0.007 b | 0.382 ± 0.009 c | 2.853 ± 0.042 c | 0.991 ± 0.007 c | 0.862 ± 0.008 c | |
| 50% EtOH | 25.34 | 10.333 ± 0.082 d | 0.190 ± 0.006 c | 0.480 ± 0.011 d | 3.505 ± 0.059 d | 1.081 ± 0.008 c | 0.913 ± 0.003 c | |
| DV2 | 96 °GL EtOH | 11.14 | ND | 0.140 ± 0.002 b | 0.164 ± 0.004 a | 0.577 ± 0.002 e | 0.570 ± 0.001 a | 0.595 ± 0.001 a |
| 90% EtOH | 14.40 | 2.311 ± 0.106 e | 0.261 ± 0.003 d | 0.743 ± 0.015 e | 2.408 ± 0.060 c | 0.798 ± 0.007 b | 0.944 ± 0.005 c | |
| 70% EtOH | 19.13 | 1.263 ± 0.036 f | 0.141 ± 0.001 b | 0.082 ± 0.004 f | 0.740 ± 0.005 a | 0.992 ± 0.003 c | 0.743 ± 0.004 b | |
| 50% EtOH | 22.58 | 1.244 ± 0.020 f | 0.083 ± 0.009 a | 0.172 ± 0.004 a | 0.572 ± 0.001 e | 1.089 ± 0.009 c | 0.690 ±0.004 b | |
| DVMus | 96 °GL EtOH | 4.07 | ND | ND | 0.181 ± 0.004 a | ND | ND | 0.578 ± 0.010 a |
| 90% EtOH | 19.65 | 0.762 ± 0.030 g | 0.046 ± 0.008 a | 0.312 ± 0.015 c | 0.612 ± 0.005 e | 0.581 ± 0.018 a | 0.607 ± 0.008 a | |
| 70% EtOH | 21.51 | 7.473 ± 0.027 h | 0.020 ± 0.004 a | 0.235 ± 0.005 b | ND | 0.574 ± 0.004 a | 0.585 ± 0.012 a | |
| 50% EtOH | 22.21 | 7.595 ± 0.080 h | ND | 0.244 ± 0.007 b | 0.576 ± 0.001 e | 0.574 ± 0.001 a | 0.581 ± 0,013 a | |
| EG | 96 °GL EtOH | 2.97 | ND | 0.130 ± 0.001 a | 1.432 ± 0.063 g | 0.718 ± 0.005 a | 0.620 ± 0.002 a | 0.630 ± 0.034 a |
| 90% EtOH | 11.37 | ND | 0.262 ± 0.005 d | 2.742 ± 0.130 h | 1.320 ± 0.050 b | 0.852 ± 0.017 b | 0.831 ± 0.026 c | |
| 70% EtOH | 22.13 | 1.731 ± 0.063 i | 0.352 ± 0.006 e | 3.701 ± 0.022 i | 4.471 ± 0.087 f | 1.262 ± 0.008 c | 0.852 ± 0.014 c | |
| 50% EtOH | 22.76 | 20.123 ± 0.161 j | 0.734 ± 0.007 f | 0.452 ± 0.017 d | 6.762 ± 0.118 g | 1.893± 0.005 c | 1.134 ± 0.017 d | |
| Plant Drug | Extract | DPPH (EC50 μg/mL ± SD) | β-Carotene (IC50 μg/mL ± SD) | ROS (% Reduction) |
|---|---|---|---|---|
| DV1 | 96 °GL EtOH | >200 a | >200 a | NA |
| 90% EtOH | 140.2 ± 1.5 b | 44.4 ± 1.6 b | NA | |
| 70% EtOH | 139.2 ± 1.8 b | 41.7 ± 1.5 b | NA | |
| 50% EtOH | 109.6 ± 1.7 c | 40.1 ± 1.6 b | 9.1 ± 5.5 a | |
| DV2 | 96 °GL EtOH | >200 a | >200 a | NA |
| 90% EtOH | >200 a | >200 a | NA | |
| 70% EtOH | >200 a | >200 a | NA | |
| 50% EtOH | >200 a | 23.9 ± 1.8 c | 14.8 ± 3.4 b | |
| DVMus | 96 °GL EtOH | 180.8 ± 1.5 d | 22.6 ±1.6 c | NA |
| 90% EtOH | >200 a | 36.0 ± 1.9 b | NA | |
| 70% EtOH | >200 a | 32.8 ± 1.5 b | NA | |
| 50% EtOH | >200 a | 17.7 ± 1.5 d | 16.3 ± 2.2 c | |
| EG | 96 °GL EtOH | >200 a | 8.7 ±1.5 e | NA |
| 90% EtOH | >200 a | 11.9 ± 1.4 f | NA | |
| 70% EtOH | 135.2 ± 1.9 b | 8.3 ± 1.1 e | NA | |
| 50% EtOH | >200 a | 6.8 ± 1.5 g | 19.0 ± 6.2 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, M.P.; da Silva, L.M.C.; Silva, L.P.M.; Gomes, J.H.d.S.; Pádua, R.M.d.; Batista, J.A.N.; Sena, M.M.; Campana, P.R.V.; Braga, F.C. Quantitative Chemical Composition, Anti-Oxidant Activity, and Inhibition of TNF Release by THP-1 Cells Induced by Extracts of Echinodorus macrophyllus and Echinodorus grandiflorus. Antioxidants 2023, 12, 1365. https://doi.org/10.3390/antiox12071365
Rocha MP, da Silva LMC, Silva LPM, Gomes JHdS, Pádua RMd, Batista JAN, Sena MM, Campana PRV, Braga FC. Quantitative Chemical Composition, Anti-Oxidant Activity, and Inhibition of TNF Release by THP-1 Cells Induced by Extracts of Echinodorus macrophyllus and Echinodorus grandiflorus. Antioxidants. 2023; 12(7):1365. https://doi.org/10.3390/antiox12071365
Chicago/Turabian StyleRocha, Marina Pereira, Lyandra Maciel Cabral da Silva, Laura Paulino Maia Silva, José Hugo de Sousa Gomes, Rodrigo Maia de Pádua, João Aguiar Nogueira Batista, Marcelo Martins Sena, Priscilla Rodrigues Valadares Campana, and Fernão Castro Braga. 2023. "Quantitative Chemical Composition, Anti-Oxidant Activity, and Inhibition of TNF Release by THP-1 Cells Induced by Extracts of Echinodorus macrophyllus and Echinodorus grandiflorus" Antioxidants 12, no. 7: 1365. https://doi.org/10.3390/antiox12071365
APA StyleRocha, M. P., da Silva, L. M. C., Silva, L. P. M., Gomes, J. H. d. S., Pádua, R. M. d., Batista, J. A. N., Sena, M. M., Campana, P. R. V., & Braga, F. C. (2023). Quantitative Chemical Composition, Anti-Oxidant Activity, and Inhibition of TNF Release by THP-1 Cells Induced by Extracts of Echinodorus macrophyllus and Echinodorus grandiflorus. Antioxidants, 12(7), 1365. https://doi.org/10.3390/antiox12071365