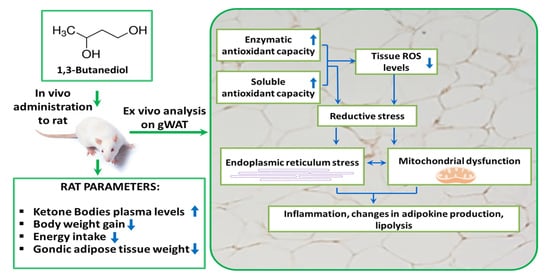
1,3-Butanediol Administration Increases β-Hydroxybutyrate Plasma Levels and Affects Redox Homeostasis, Endoplasmic Reticulum Stress, and Adipokine Production in Rat Gonadal Adipose Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Histological Analysis
2.3. Lipolysis
2.4. Western Blot
2.5. Determination of gWAT ROS Levels, Lipid and Protein Oxidative Damages, and Total Antioxidant Capacities
2.6. Adipokines Content Analysis
2.7. Statistical Analysis
3. Results
3.1. BD Administration Reduces Epididymal gWAT Mass and Adipocyte Size and Promotes Lipolysis
3.2. Effect of BD on Redox Homeostasis
3.3. Effect of BD on gWAT ER Stress and Inflammation
3.4. Effect of BD on gWAT Oxidative Capacity and Mitochondrial Respiration Complexes Abundance
3.5. Administration of BD for 14 Days Affects Specific gWAT Adipokines/Cytokines Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puchalska, P.; Crawford, P.A. Metabolic and Signaling Roles of Ketone Bodies in Health and Disease. Annu. Rev. Nutr. 2021, 41, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Cogan, K.E.; Egan, B. Metabolism of ketone bodies during exercise and training: Physiological basis for exogenous supplementation. J. Physiol. 2017, 595, 2857–2871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, J.C.; Verdin, E. β-hydroxybutyrate: Much more than a metabolite. Diabetes Res. Clin. Pract. 2014, 106, 173–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas-Morales, P.; Tapia, E.; Pedraza-Chaverri, J. β-Hydroxybutyrate: A signaling metabolite in starvation response? Cell Signal. 2016, 28, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef]
- Taggart, A.K.; Kero, J.; Gan, X.; Cai, T.Q.; Cheng, K.; Ippolito, M.; Ren, N.; Kaplan, R.; Wu, K.; Wu, T.J.; et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005, 280, 26649–26652. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, J.; Ohue-Kitano, R.; Mukouyama, H.; Nishida, A.; Watanabe, K.; Igarashi, M.; Irie, J.; Tsujimoto, G.; Satoh-Asahara, N.; Itoh, H.; et al. Ketone body receptor GPR43 regulates lipid metabolism under ketogenic conditions. Proc. Natl. Acad. Sci. USA 2019, 116, 23813–23821. [Google Scholar] [CrossRef] [Green Version]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [Green Version]
- McNally, M.A.; Hartman, A.L. Ketone bodies in epilepsy. J. Neurochem. 2012, 121, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Shan, W.; Zhu, F.; Wu, J.; Wang, Q. Ketone Bodies in Neurological Diseases: Focus on Neuroprotection and Underlying Mechanisms. Front. Neurol. 2019, 10, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishitani, S.; Fukuhara, A.; Tomita, I.; Kume, S.; Shin, J.; Okuno, Y.; Otsuki, M.; Maegawa, H.; Shimomura, I. Ketone body 3-hydroxybutyrate enhances adipocyte function. Sci. Rep. 2022, 12, 10080. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, R.; Cipriani, F.; Masi, D.; Basciani, S.; Watanabe, M.; Lubrano, C.; Gnessi, L.; Mariani, S. Ketone Bodies and SIRT1, Synergic Epigenetic Regulators for Metabolic Health: A Narrative Review. Nutrients 2022, 14, 3145. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. Adipose Organ Development and Remodeling. Compr. Physiol. 2018, 8, 1357–1431. [Google Scholar] [CrossRef]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Jankovic, A.; Korac, A.; Buzadzic, B.; Otasevic, V.; Stancic, A.; Daiber, A.; Korac, B. Redox implications in adipose tissue (dys)function-A new look at old acquaintances. Redox Biol. 2015, 6, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Liu, S.; Zou, L.; Xu, C.; Geng, B.; Xu, G. Lipolysis response to endoplasmic reticulum stress in adipose cells. J. Biol. Chem. 2012, 287, 6240–6249. [Google Scholar] [CrossRef] [Green Version]
- Dandekar, A.; Mendez, R.; Zhang, K. Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol. Biol. 2015, 1292, 205–214. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.S.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Rzedox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef] [Green Version]
- Qiao, X.; Zhang, Y.; Ye, A.; Zhang, Y.; Xie, T.; Lv, Z.; Shi, C.; Wu, D.; Chu, B.; Wu, X.; et al. ER reductive stress caused by Ero1α S-nitrosation accelerates senescence. Free Radic. Biol. Med. 2022, 180, 165–178. [Google Scholar] [CrossRef]
- Lim, J.H.; Lee, H.J.; Ho Jung, M.; Song, J. Coupling mitochondrial dysfunction to endoplasmic reticulum stress response: A molecular mechanism leading to hepatic insulin resistance. Cell Signal. 2009, 21, 169–177. [Google Scholar] [CrossRef]
- Lionetti, L.; Mollica, M.P.; Lombardi, A.; Cavaliere, G.; Gifuni, G.; Barletta, A. From chronic overnutrition to insulin resistance: The role of fat-storing capacity and inflammation. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Després, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef]
- Bjørndal, B.; Burri, L.; Staalesen, V.; Skorve, J.; Berge, R.K. Different adipose depots: Their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J. Obes. 2011, 2011, 490650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poff, A.M.; Rho, J.M.; D’Agostino, D.P. Ketone Administration for Seizure Disorders: History and Rationale for Ketone Esters and Metabolic Alternatives. Front. Neurosci. 2019, 13, 1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Agostino, D.P.; Pilla, R.; Held, H.E.; Landon, C.S.; Puchowicz, M.; Brunengraber, H.; Ari, C.; Arnold, P.; Dean, J.B. Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R829–R836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langhans, W.; Wiesenreiter, F.; Scharrer, E. Different effects of subcutaneous D,L-3-hydroxybutyrate and acetoacetate injections on food intake in rats. Physiol. Behav. 1983, 31, 483–486. [Google Scholar] [CrossRef]
- Langhans, W.; Pantel, K.; Scharrer, E. Ketone kinetics and D-(−)-3-hydroxybutyrate-induced inhibition of feeding in rats. Physiol. Behav. 1985, 34, 579–582. [Google Scholar] [CrossRef]
- Gentile, A.; Magnacca, N.; de Matteis, R.; Moreno, M.; Cioffi, F.; Giacco, A.; Lanni, A.; de Lange, P.; Senese, R.; Goglia, F.; et al. Ablation of uncoupling protein 3 affects interrelated factors leading to lipolysis and insulin resistance in visceral white adipose tissue. FASEB J. 2022, 36, e22325. [Google Scholar] [CrossRef]
- Clark, A.M.; Sousa, K.M.; Jennings, C.; MacDougald, O.A.; Kennedy, R.T. Continuous-flow enzyme assay on a microfluidic chip for monitoring glycerol secretion from cultured adipocytes. Anal. Chem. 2009, 81, 2350–2356. [Google Scholar] [CrossRef] [Green Version]
- Driver, A.S.; Kodavanti, P.R.; Mundy, W.R. Age-related changes in reactive oxygen species production in rat brain homogenates. Neurotoxicol. Teratol. 2000, 22, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Tappel, A.L. A new sensitive assay for the measurement of hydroperoxides. Anal. Biochem. 1976, 76, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Norwitz, N.G.; Hu, M.T.; Clarke, K. The Mechanisms by Which the Ketone Body D-β-Hydroxybutyrate May Improve the Multiple Cellular Pathologies of Parkinson’s Disease. Front. Nutr. 2019, 6, 63. [Google Scholar] [CrossRef]
- Sato, K.; Kashiwaya, Y.; Keon, C.A.; Tsuchiya, N.; King, M.T.; Radda, G.K.; Chance, B.; Clarke, K.; Veech, R.L. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995, 9, 651–658. [Google Scholar] [CrossRef]
- Agledal, L.; Niere, M.; Ziegler, M. The phosphate makes a difference: Cellular functions of NADP. Redox Rep. 2010, 15, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Blacker, T.S.; Duchen, M.R. Investigating mitochondrial redox state using NADH and NADPH autofluorescence. Free Radic. Biol. Med. 2016, 100, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Veech, R.L.; Bradshaw, P.C.; Clarke, K.; Curtis, W.; Pawlosky, R.; King, M.T. Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life 2017, 69, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Kolb, H.; Kempf, K.; Röhling, M.; Lenzen-Schulte, M.; Schloot, N.C.; Martin, S. Ketone bodies: From enemy to friend and guardian angel. BMC Med. 2021, 19, 313. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Guarner-Lans, V.; Rubio-Ruiz, M.E. Reductive Stress in Inflammation-Associated Diseases and the Pro-Oxidant Effect of Antioxidant Agents. Int. J. Mol. Sci. 2017, 18, 2098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef] [Green Version]
- Singh, F.; Charles, A.L.; Schlagowski, A.I.; Bouitbir, J.; Bonifacio, A.; Piquard, F.; Krähenbühl, S.; Geny, B.; Zoll, J. Reductive stress impairs myoblasts mitochondrial function and triggers mitochondrial hormesis. Biochim. Biophys. Acta 2015, 1853, 1574–1585. [Google Scholar] [CrossRef] [Green Version]
- Peris, E.; Micallef, P.; Paul, A.; Palsdottir, V.; Enejder, A.; Bauzá-Thorbrügge, M.; Olofsson, C.S.; Wernstedt Asterholm, I. Antioxidant treatment induces reductive stress associated with mitochondrial dysfunction in adipocytes. J. Biol. Chem. 2019, 294, 2340–2352. [Google Scholar] [CrossRef] [Green Version]
- Gregor, M.F.; Hotamisligil, G.S. Thematic review series: Adipocyte Biology. Adipocyte stress: The endoplasmic reticulum and metabolic disease. J. Lipid Res. 2007, 48, 1905–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdanovic, E.; Kraus, N.; Patsouris, D.; Diao, L.; Wang, V.; Abdullahi, A.; Jeschke, M.G. Endoplasmic reticulum stress in adipose tissue augments lipolysis. J. Cell. Mol. Med. 2015, 19, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Rydén, M.; Arvidsson, E.; Blomqvist, L.; Perbeck, L.; Dicker, A.; Arner, P. Targets for TNF-alpha-induced lipolysis in human adipocytes. Biochem. Biophys. Res. Commun. 2004, 318, 168–175. [Google Scholar] [CrossRef]
- Mondal, A.K.; Das, S.K.; Varma, V.; Nolen, G.T.; McGehee, R.E.; Elbein, S.C.; Wei, J.Y.; Ranganathan, G. Effect of endoplasmic reticulum stress on inflammation and adiponectin regulation in human adipocytes. Metab. Syndr. Relat. Disord. 2012, 10, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Isoda, M.; Ebihara, K.; Sawayama, N.; Murakami, A.; Ebihara, C.; Shibuya, K.; Takei, A.; Takei, S.; Wakabayashi, T.; Yamamuro, D.; et al. Leptin sensitizing effect of 1,3-butanediol and its potential mechanism. Sci. Rep. 2021, 11, 17691. [Google Scholar] [CrossRef]
- Keophiphath, M.; Rouault, C.; Divoux, A.; Clément, K.; Lacasa, D. CCL5 promotes macrophage recruitment and survival in human adipose tissue. Arter. Thromb. Vasc. Biol. 2010, 30, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, L.; Liu, Z.; Liu, Y.; Luo, M.; Chen, N.; Deng, X.; Luo, Y.; He, J.; Zhang, L.; et al. PAI-1 Exacerbates White Adipose Tissue Dysfunction and Metabolic Dysregulation in High Fat Diet-Induced Obesity. Front. Pharmacol. 2018, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhu, L.; Wu, J. RAGE signalling in obesity and diabetes: Focus on the adipose tissue macrophage. Adipocyte 2020, 9, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Juge-Aubry, C.E.; Somm, E.; Pernin, A.; Alizadeh, N.; Giusti, V.; Dayer, J.M.; Meier, C.A. Adipose tissue is a regulated source of interleukin-10. Cytokine 2005, 29, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Pepper, M.S.; Montesano, R.; Mandriota, S.J.; Orci, L.; Vassalli, J.D. Angiogenesis: A paradigm for balanced extracellular proteolysis during cell migration and morphogenesis. Enzym. Protein 1996, 49, 138–162. [Google Scholar] [CrossRef]
- Pepper, M.S. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arter. Thromb. Vasc. Biol. 2001, 21, 1104–1117. [Google Scholar] [CrossRef] [Green Version]
- Frühbeck, G.; Méndez-Giménez, L.; Fernández-Formoso, J.A.; Fernández, S.; Rodríguez, A. Regulation of adipocyte lipolysis. Nutr. Res. Rev. 2014, 27, 63–93. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, C.G.; Jia, Y.L.; Hu, L. Tissue Inhibitor of Metalloprotease-1 (TIMP-1) Regulates Adipogenesis of Adipose-derived Stem Cells (ASCs) via the Wnt Signaling Pathway in an MMP-independent Manner. Curr. Med. Sci. 2020, 40, 989–996. [Google Scholar] [CrossRef]
- Liang, X.; Kanjanabuch, T.; Mao, S.L.; Hao, C.M.; Tang, Y.W.; Declerck, P.J.; Hasty, A.H.; Wasserman, D.H.; Fogo, A.B.; Ma, L.J. Plasminogen activator inhibitor-1 modulates adipocyte differentiation. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E103–E113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudak, C.S.; Sul, H.S. Pref-1, a gatekeeper of adipogenesis. Front. Endocrinol. 2013, 4, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suchacki, K.J.; Thomas, B.J.; Ikushima, Y.M.; Chen, K.C.; Fyfe, C.; Tavares, A.A.S.; Sulston, R.J.; Lovdel, A.; Woodward, H.J.; Han, X.; et al. The effects of caloric restriction on adipose tissue and metabolic health are sex- and age-dependent. Elife 2023, 12, e88080. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.L.; Zhang, W.; Gao, X.; Watts, L. Caloric restriction increases ketone bodies metabolism and preserves blood flow in aging brain. Neurobiol. Aging 2015, 36, 2296–2303. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| C | BD 14 d | |
|---|---|---|
| Body weight at the beginning of the treatment (g) | 273 ± 7 | 271 ± 4 |
| Body weight at the end of the treatment (g) | 329 ± 6 | 300 ± 7 ** |
| Body weight gain (g) | 55 ± 3 | 29 ± 4 *** |
| Energy intake (kJ) | 3840 ± 363 | 2828 ± 348 *** |
| gWAT weight (g) | 4.5 ± 0.4 | 3.2 ± 0.1 ** |
| Glucose serum levels (mg/dL) | 112 ± 6 | 120 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panico, G.; Fasciolo, G.; Migliaccio, V.; De Matteis, R.; Lionetti, L.; Napolitano, G.; Agnisola, C.; Venditti, P.; Lombardi, A. 1,3-Butanediol Administration Increases β-Hydroxybutyrate Plasma Levels and Affects Redox Homeostasis, Endoplasmic Reticulum Stress, and Adipokine Production in Rat Gonadal Adipose Tissue. Antioxidants 2023, 12, 1471. https://doi.org/10.3390/antiox12071471
Panico G, Fasciolo G, Migliaccio V, De Matteis R, Lionetti L, Napolitano G, Agnisola C, Venditti P, Lombardi A. 1,3-Butanediol Administration Increases β-Hydroxybutyrate Plasma Levels and Affects Redox Homeostasis, Endoplasmic Reticulum Stress, and Adipokine Production in Rat Gonadal Adipose Tissue. Antioxidants. 2023; 12(7):1471. https://doi.org/10.3390/antiox12071471
Chicago/Turabian StylePanico, Giuliana, Gianluca Fasciolo, Vincenzo Migliaccio, Rita De Matteis, Lillà Lionetti, Gaetana Napolitano, Claudio Agnisola, Paola Venditti, and Assunta Lombardi. 2023. "1,3-Butanediol Administration Increases β-Hydroxybutyrate Plasma Levels and Affects Redox Homeostasis, Endoplasmic Reticulum Stress, and Adipokine Production in Rat Gonadal Adipose Tissue" Antioxidants 12, no. 7: 1471. https://doi.org/10.3390/antiox12071471
APA StylePanico, G., Fasciolo, G., Migliaccio, V., De Matteis, R., Lionetti, L., Napolitano, G., Agnisola, C., Venditti, P., & Lombardi, A. (2023). 1,3-Butanediol Administration Increases β-Hydroxybutyrate Plasma Levels and Affects Redox Homeostasis, Endoplasmic Reticulum Stress, and Adipokine Production in Rat Gonadal Adipose Tissue. Antioxidants, 12(7), 1471. https://doi.org/10.3390/antiox12071471