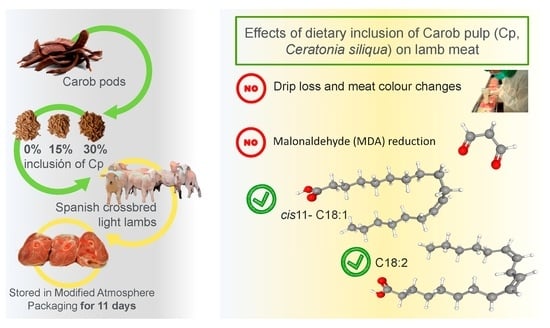
Colour Stability, Fatty Acid Profile, and Lipid Oxidation in Meat Stored in Modified Atmosphere Packaging from Light Lambs Fed with Concentrate with Carob Pulp (Ceratonia siliqua L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diets, Slaughter Procedures, and Meat Sampling
2.2. Thawing and Drip Loss, Moisture Content, and Colour Stability Measurements
2.3. Analysis of Fatty Acid Composition in Feedstuffs and Semimembranosus Muscle Sample
2.4. Analysis of Cholesterol, Tocopherol Isomers, and Total Polyphenols
2.5. Lipid Oxidation Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Thawed and Drip Losses and Meat Colour Evolution
3.2. Fatty Acid Profile in Semimembranosus Muscle
3.3. Tocopherol and Cholesterol Contents in the Semimembranosus Muscle
3.4. Lipid Oxidation
3.5. Relationship between α-Tocopherol Meat Content and Lipid Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential Oils as Natural Additives to Prevent Oxidation Reactions in Meat and Meat Products: A Review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Rubio, B.; Vieira, C.; Martínez, B. Effect of Post Mortem Temperatures and Modified Atmospheres Packaging on Shelf Life of Suckling Lamb Meat. LWT Food Sci. Technol. 2016, 69, 563–569. [Google Scholar] [CrossRef]
- Luo, Y.; Ju, N.; Chang, J.; Ge, R.; Zhao, Y.; Zhang, G. Dietary α-Lipoic Acid Supplementation Improves Postmortem Color Stability of the Lamb Muscles through Changing Muscle Fiber Types and Antioxidative Status. Meat Sci. 2022, 193, 108945. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Romano, A.; Moreno-Rojas, J.M. Carob Pulp: A Nutritional and Functional By-Product. Processes 2021, 9, 1146. [Google Scholar] [CrossRef]
- Priolo, A.; Waghorn, G.C.; Lanza, M.; Biondi, L.; Pennisi, P. Polyethylene Glycol as a Means for Reducing the Impact of Condensed Tannins in Carob Pulp: Effects on Lamb Growth Performance and Meat Quality. J. Anim. Sci. 2000, 78, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Frutos, P.; Hervás, G.; Natalello, A.; Luciano, G.; Fondevila, M.; Priolo, A.; Toral, P.G. Ability of Tannins to Modulate Ruminal Lipid Metabolism and Milk and Meat Fatty Acid Profiles. Anim. Feed Sci. Technol. 2020, 269, 114623. [Google Scholar] [CrossRef]
- Luciano, G.; Monahan, F.J.; Vasta, V.; Biondi, L.; Lanza, M.; Priolo, A. Dietary Tannins Improve Lamb Meat Colour Stability. Meat Sci. 2009, 81, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Lobón, S.; Blanco, M.; Sanz, A.; Ripoll, G.; Bertolín, J.R.; Joy, M. Meat Quality of Light Lambs Is More Affected by the Dam’s Feeding System during Lactation than by the Inclusion of Quebracho in the Fattening Concentrate. J. Anim. Sci. 2017, 95, 4998–5011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, R.N.S.; Ghedini, C.P.; Paschoaloto, J.R.; da Silva, D.A.V.; Coelho, L.M.; Almeida Junior, G.A.; Ezequiel, J.M.B.; Machado Neto, O.R.; Almeida, M.T.C. Effects of Tannins Supplementation to Sheep Diets on Their Performance, Carcass Parameters and Meat Fatty Acid Profile: A Meta-Analysis Study. Small Rumin. Res. 2022, 206, 17. [Google Scholar] [CrossRef]
- Vasta, V.; Mele, M.; Serra, A.; Scerra, M.; Luciano, G.; Lanza, M.; Priolo, A. Metabolic Fate of Fatty Acids Involved in Ruminal Biohydrogenation in Sheep Fed Concentrate or Herbage with or without Tannins. J. Anim. Sci. 2009, 87, 2674–2684. [Google Scholar] [CrossRef] [Green Version]
- Priolo, A.; Vasta, V.; Fasone, V.; Lanza, C.M.; Scerra, M.; Biondi, L.; Bella, M.; Whittington, F.M. Meat Odour and Flavour and Indoles Concentration in Ruminal Fluid and Adipose Tissue of Lambs Fed Green Herbage or Concentrates with or without Tannins. Animal 2009, 3, 454–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo, J.M.; Trindade, M.A.; Ahn, D.U.; Barba, F.J. Natural Antioxidants to Reduce the Oxidation Process of Meat and Meat Products. Food Res. Int. 2019, 115, 377–378. [Google Scholar] [CrossRef] [PubMed]
- Ponnampalam, E.N.; Kiani, A.; Santhiravel, S.; Holman, B.W.B.; Lauridsen, C.; Dunshea, F.R. The Importance of Dietary Antioxidants on Oxidative Stress, Meat and Milk Production, and Their Preservative Aspects in Farm Animals: Antioxidant Action, Animal Health, and Product Quality—Invited Review. Animals 2022, 12, 3279. [Google Scholar] [CrossRef]
- Vasta, V.; Luciano, G. The Effects of Dietary Consumption of Plants Secondary Compounds on Small Ruminants’ Products Quality. Small Rumin. Res. 2011, 101, 150–159. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of Dietary Tannins to Improve Rumen Metabolism and Ruminant Nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited Review: Plant Polyphenols and Rumen Microbiota Responsible for Fatty Acid Biohydrogenation, Fiber Digestion, and Methane Emission: Experimental Evidence and Methodological Approaches. J. Dairy Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef]
- Pelegrin-Valls, J.; Serrano-Pérez, B.; Villalba, D.; Molina, E.; Espinal, J.; Joy, M.; Álvarez-Rodríguez, J. Is the Inclusion of Carob (Ceratonia siliqua L.) Pulp in the Concentrate of Weaned Light Lambs Worth It? Anim. Feed Sci. Technol. 2022, 293, 115452. [Google Scholar] [CrossRef]
- Association American Meat Science. AMSA Meat Color Measurement Guidelines; Association American Meat Science: Savoy, IL, USA, 2012; Volume 12, ISBN 8005172672. [Google Scholar]
- Christensen, L.B. Drip Loss Sampling in Porcine m. Longissimus Dorsi. Meat Sci. 2003, 63, 469–477. [Google Scholar] [CrossRef]
- Kristensen, L.; Purslow, P.P. The Effect of Ageing on the Water-Holding Capacity of Pork: Role of Cytoskeletal Proteins. Meat Sci. 2001, 58, 17–23. [Google Scholar] [CrossRef]
- Sukhija, P.S.; Palmquist, D.L. Rapid Method for Determination of Total Fatty Acid Content and Composition of Feedstuffs and Feces. J. Agric. Food Chem. 1988, 36, 1202–1206. [Google Scholar] [CrossRef]
- Lee, M.R.F.; Tweed, J.K.S.; Kim, E.J.; Scollan, N.D. Beef, Chicken and Lamb Fatty Acid Analysis—A Simplified Direct Bimethylation Procedure Using Freeze-Dried Material. Meat Sci. 2012, 92, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.P.; Bessa, R.J.B. Comparison of Two Gas-Liquid Chromatograph Columns for the Analysis of Fatty Acids in Ruminant Meat. J. Chromatogr. A 2009, 1216, 5130–5139. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Lamas, L.; Barron, L.J.R.; Kramer, J.K.G.; Etaio, I.; Aldai, N. Characterization of the Fatty Acid Composition of Lamb Commercially Available in Northern Spain: Emphasis on the Trans-18:1 and CLA Content and Profile. Meat Sci. 2016, 117, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.K.G.; Fellner, V.; Dugan, M.E.R.; Sauer, F.D.; Mossoba, M.M.; Yurawecz, M.P. Evaluating Acid and Base Catalysts in the Methylation of Milk and Rumen Fatty Acids with Special Emphasis on Conjugated Dienes and Total Trans Fatty Acids. Lipids 1997, 32, 1219–1228. [Google Scholar] [CrossRef]
- ISO 12966-4:2015; Animal and Vegetables Fat and Oils. Gas Chromatography of Fatty Acid Methyl Esters. Part 4: Determination by Capillary Chromatography. International Organization for Standardization: Geneva, Switzerland.
- AOAC (Association of Analytical Chemists). Official Method 960.39 Fat (Crude) or Ether Extract in Meat. In Official Methods of Analysis, 17th ed.; AOAC (Association of Analytical Chemists): Arlington, VA, USA, 2000. [Google Scholar]
- Blanco, M.; Ripoll, G.; Casasús, I.; Bertolín, J.R.; Joy, M. Carotenoids and Tocopherol in Plasma and Subcutaneous Fat Colour to Trace Forage-Feeding in Growing Steers. Livest. Sci. 2019, 219, 104–110. [Google Scholar] [CrossRef]
- Bertolín, J.R.; Joy, M.; Rufino-Moya, P.J.; Lobón, S.; Blanco, M. Simultaneous Determination of Carotenoids, Tocopherols, Retinol and Cholesterol in Ovine Lyophilised Samples of Milk, Meat, and Liver and in Unprocessed/Raw Samples of Fat. Food Chem. 2018, 257, 182–188. [Google Scholar] [CrossRef]
- Rufino-Moya, P.J.; Blanco, M.; Bertolín, J.R.; Joy, M. Effect of the Method of Preservation on the Chemical Composition and in Vitro Fermentation Characteristics in Two Legumes Rich in Condensed Tannins. Anim. Feed Sci. Technol. 2019, 251, 12–20. [Google Scholar] [CrossRef]
- Bertolín, J.R.; Joy, M.; Blanco, M. Malondialdehyde Determination in Raw and Processed Meat Products by UPLC-DAD and UPLC-FLD. Food Chem. 2019, 298, 125009. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. Infostat Version; Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2020. [Google Scholar]
- Kim, Y.H.B.; Liesse, C.; Kemp, R.; Balan, P. Evaluation of Combined Effects of Ageing Period and Freezing Rate on Quality Attributes of Beef Loins. Meat Sci. 2015, 110, 40–45. [Google Scholar] [CrossRef]
- Testa, M.L.; Grigioni, G.; Panea, B.; Pavan, E.; Sandell, M. Color and Marbling as Predictors of Meat Quality Perception of Argentinian Consumers. Foods 2021, 10, 1465. [Google Scholar] [CrossRef]
- Gravador, R.S.; Luciano, G.; Jongberg, S.; Bognanno, M.; Scerra, M.; Andersen, M.L.; Lund, M.N.; Priolo, A. Fatty Acids and Oxidative Stability of Meat from Lambs Fed Carob-Containing Diets. Food Chem. 2015, 182, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Yagoubi, Y.; Joy, M.; Ripoll, G.; Mahouachi, M.; Bertolín, J.R.; Atti, N. Rosemary Distillation Residues Reduce Lipid Oxidation, Increase Alpha-Tocopherol Content and Improve Fatty Acid Profile of Lamb Meat. Meat Sci. 2018, 136, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Díaz, M.T.; Álvarez, I.; De La Fuente, J.; Sañudo, C.; Campo, M.M.; Oliver, M.A.; Font I Furnols, M.; Montossi, F.; San Julián, R.; Nute, G.R.; et al. Fatty Acid Composition of Meat from Typical Lamb Production Systems of Spain, United Kingdom, Germany and Uruguay. Meat Sci. 2005, 71, 256–263. [Google Scholar] [CrossRef] [PubMed]
- FEDNA. Necessidades Nutricionales Para Rumiantes En Cebo: Normas FEDNA; FEDNA: Madrid, Spain, 2008; ISBN 13-978-84-612-7157-3. [Google Scholar]
- Thompson, J.M. The Effects of Marbling on Flavour and Juiciness Scores of Cooked Beef, after Adjusting to a Constant Tenderness. Aust. J. Exp. Agric. 2004, 44, 645–652. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, J.; Villalba, D.; Molina, E.; Serrano-Pérez, B.; Bertolín, J.R.; Joy, M. ¿Afectan Los Taninos Condensados de La Dieta a Los Resultados Productivos, La Composición de Ácidos Grasos y El Color de La Carne de Cordero? Inf. Tec. Econ. Agrar. 2020, 116, 116–130. [Google Scholar] [CrossRef]
- Guerreiro, O.; Alves, S.P.; Costa, M.; Duarte, M.F.; Jerónimo, E.; Bessa, R.J.B. Effects of Increasing Doses of Condensed Tannins Extract from Cistus ladanifer L. on In Vitro Ruminal Fermentation and Biohydrogenation. Animals 2021, 11, 761. [Google Scholar] [CrossRef]
- Watkins, P.J.; Jaborek, J.R.; Teng, F.; Day, L.; Castada, H.Z.; Baringer, S.; Wick, M. Branched Chain Fatty Acids in the Flavour of Sheep and Goat Milk and Meat: A Review. Small Rumin. Res. 2021, 200, 106398. [Google Scholar] [CrossRef]
- Fievez, V.; Colman, E.; Castro-Montoya, J.M.; Stefanov, I.; Vlaeminck, B. Milk Odd-and Branched-Chain Fatty Acids as Biomarkers of Rumen Function-An Update. Anim. Feed Sci. Technol. 2012, 172, 51–65. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; Galisteo, O.O.; Ramírez, C.A.; Blanco, F.P.; De La Fuente, M.A.; Sánchez, N.N.; Marín, A.L.M. Intramuscular Fatty Acid Profile of Feedlot Lambs Fed Concentrates with Alternative Ingredients. Anim. Prod. Sci. 2019, 59, 914–920. [Google Scholar] [CrossRef]
- Natalello, A.; Luciano, G.; Morbidini, L.; Valenti, B.; Pauselli, M.; Frutos, P.; Biondi, L.; Rufino-Moya, P.J.; Lanza, M.; Priolo, A. Effect of Feeding Pomegranate Byproduct on Fatty Acid Composition of Ruminal Digesta, Liver, and Muscle in Lambs. J. Agric. Food Chem. 2019, 67, 4472–4482. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Z.; Li, X.; Zhu, B.; Guo, L.; Li, F.; Weng, X. Effect of Duration of Linseed Diet Supplementation before Slaughter on the Performances, Meat Fatty Acid Composition and Rumen Bacterial Community of Fattening Lambs. Anim. Feed. Sci. Technol. 2020, 263, 114457. [Google Scholar] [CrossRef]
- Hampel, V.S.; Poli, C.H.E.C.; Joy, M.; Tontini, J.F.; Devincenzi, T.; Pardos, J.R.B.; Macedo, R.E.F.; Nalério, E.N.; Saccol, A.G.F.; Rodrigues, E.; et al. Tropical Grass and Legume Pastures May Alter Lamb Meat Physical and Chemical Characteristics. Trop. Anim. Health Prod. 2021, 1, 427. [Google Scholar] [CrossRef]
- Bessa, R.J.B.; Alves, S.P.; Santos-Silva, J. Constraints and Potentials for the Nutritional Modulation of the Fatty Acid Composition of Ruminant Meat. Eur. J. Lipid Sci. Technol. 2015, 117, 1325–1344. [Google Scholar] [CrossRef]
- De Blas, C.; García-Rebollar, P.; Gorrachategui, M.; Mateos, G.G. Tablas FEDNA de Composición y Valor Nutritivo de Alimentos Para la Fabricación de Alimentos Compuestos, 4th ed.; Fundación Española para el Desarrollo de la Nutrición Animal: Madrid, Spain, 2019; ISBN 978-8409156887. [Google Scholar]
- Kasapidou, E.; Wood, J.D.; Richardson, R.I.; Sinclair, L.A.; Wilkinson, R.G.; Enser, M. Effect of Vitamin E Supplementation and Diet on Fatty Acid Composition and on Meat Colour and Lipid Oxidation of Lamb Leg Steaks Displayed in Modified Atmosphere Packs. Meat Sci. 2012, 90, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Rodríguez, J.; Urrutia, O.; Lobón, S.; Ripoll, G.; Bertolín, J.R.; Joy, M. Insights into the Role of Major Bioactive Dietary Nutrients in Lamb Meat Quality: A Review. J. Anim. Sci. Biotechnol. 2022, 13, 20. [Google Scholar] [CrossRef]
- Ripoll, G.; González-Calvo, L.; Molino, F.; Calvo, J.H.; Joy, M. Effects of Finishing Period Length with Vitamin E Supplementation and Alfalfa Grazing on Carcass Color and the Evolution of Meat Color and the Lipid Oxidation of Light Lambs. Meat Sci. 2013, 93, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Lobón, S.; Sanz, A.; Blanco, M.; Ripoll, G.; Joy, M. The Type of Forage and Condensed Tannins in Dams’ Diet: Influence on Meat Shelf Life of Their Suckling Lambs. Small Rumin. Res. 2017, 154, 115–122. [Google Scholar] [CrossRef]
- Valenti, B.; Natalello, A.; Vasta, V.; Campidonico, L.; Roscini, V.; Mattioli, S.; Pauselli, M.; Priolo, A.; Lanza, M.; Luciano, G. Effect of Different Dietary Tannin Extracts on Lamb Growth Performances and Meat Oxidative Stability: Comparison between Mimosa, Chestnut and Tara. Animal 2019, 13, 435–443. [Google Scholar] [CrossRef]
- Jacondino, L.R.; Poli, C.H.E.C.; Tontini, J.F.; Corrêa, G.F.; Somacal, S.; Mello, R.O.; Leal, M.L.R.; Raimondo, R.F.S.; Riet-Correa, B.; Muir, J.P. Acacia Mearnsii Tannin Extract and α-Tocopherol Supplementation in Lamb Diet: Effects on Growth Performance, Serum Lipid Peroxidation and Meat Quality. Anim. Feed Sci. Technol. 2022, 294, 115483. [Google Scholar] [CrossRef]
- Maggiolino, A.; Bragaglio, A.; Salzano, A.; Rufrano, D.; Claps, S.; Sepe, L.; Damiano, S.; Ciarcia, R.; Dinardo, F.R.; Hopkins, D.L.; et al. Dietary Supplementation of Suckling Lambs with Anthocyanins: Effects on Growth, Carcass, Oxidative and Meat Quality Traits. Anim. Feed Sci. Technol. 2021, 276, 114925. [Google Scholar] [CrossRef]
- Ripoll, G.; Joy, M.; Muñoz, F. Use of Dietary Vitamin E and Selenium (Se) to Increase the Shelf Life of Modified Atmosphere Packaged Light Lamb Meat. Meat Sci. 2011, 87, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.; Pazos, M.; Torres, J.L.; Medina, I. Antioxidant Mechanism of Grape Procyanidins in Muscle Tissues: Redox Interactions with Endogenous Ascorbic Acid and α-Tocopherol. Food Chem. 2012, 134, 1767–1774. [Google Scholar] [CrossRef]
- González-Calvo, L.; Ripoll, G.; Molino, F.; Calvo, J.H.; Joy, M. The Relationship between Muscle α-Tocopherol Concentration and Meat Oxidation in Light Lambs Fed Vitamin E Supplements Prior to Slaughter. J. Sci. Food Agric. 2015, 95, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, I.; De La Fuente, J.; Díaz, M.T.; Lauzurica, S.; Pérez, C.; Cañeque, V. Estimation of α-Tocopherol Concentration Necessary to Optimise Lamb Meat Quality Stability during Storage in High-Oxygen Modified Atmosphere Using Broken-Line Regression Analysis. Animal 2008, 2, 1405–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Carob Pulp | C0% | C15% | C30% | Barley Straw | |
|---|---|---|---|---|---|
| Ether extract (% on DM basis) | 1.81 ± 0.05 | 2.78 ± 0.27 | 5.00 ± 0.28 | 7.20 ± 0.19 | 2.71 ± 0.08 |
| FA composition (g/100 g of FAME) | |||||
| C12:0 | 0.13 ± 0.02 | 0.18 ± 0.01 | 0.33 ± 0.02 | 0.38 ± 0.03 | 0.86 ± 0.06 |
| C14:0 | 0.96 ± 0.15 | 0.50 ± 0.01 | 0.67 ± 0.00 | 0.81 ± 0.01 | 2.82 ± 0.01 |
| C16:0 | 35.4 ± 0.25 | 25.6 ± 0.50 | 29.9 ± 0.58 | 34.9 ± 0.87 | 43.0 ± 0.52 |
| C17:0 | 0.26 ± 0.02 | 0.11 ± 0.00 | 0.11 ± 0.00 | 0.11 ± 0.00 | 0.31 ± 0.04 |
| C18:0 | 23.6 ± 2.91 | 6.40 ± 0.37 | 5.31 ± 0.38 | 5.65 ± 0.03 | 23.6 ± 2.16 |
| C20:0 | 0.76 ± 0.01 | 0.35 ± 0.02 | 0.32 ± 0.02 | 0.35 ± 0.01 | 1.32 ± 0.05 |
| C22:0 | 0.28 ± 0.05 | 0.14 ± 0.00 | 0.12 ± 0.02 | 0.10 ± 0.01 | 1.07 ± 0.13 |
| C24:0 | 0.17 ± 0.01 | 0.12 ± 0.01 | 0.09 ± 0.02 | 0.08 ± 0.01 | 0.59 ± 0.13 |
| Σ SFA | 61.6 ± 2.50 | 33.4 ± 0.90 | 36.8 ± 1.00 | 42.4 ± 0.86 | 73.6 ± 1.98 |
| cis9-C16:1 | 0.29 ± 0.41 | 0.14 ± 0.00 | 0.15 ± 0.01 | 0.16 ± 0.00 | 0.22 ± 0.00 |
| cis9-C18:1 | 26.6 ± 1.80 | 2 3.5 ± 0.15 | 28.9 ± 0.06 | 33.1 ± 0.18 | 11.3 ± 1.35 |
| cis11-C18:1 | 0.17 ± 0.10 | 0.79 ± 0.01 | 0.80 ± 0.01 | 0.80 ± 0.02 | 0.56 ± 0.05 |
| C20:1 | 0.09 ± 0.05 | 0.23 ± 0.04 | 0.17 ± 0.00 | 0.14 ± 0.00 | 0.28 ± 0.40 |
| Σ MUFA | 27.1 ± 2.26 | 24.7 ± 0.11 | 30.0 ± 0.04 | 34.2 ± 0.16 | 12.4 ± 0.90 |
| C18:2n-6 | 10.1 ± 0.23 | 39.2 ± 0.85 | 31.2 ± 0.99 | 22.1 ± 0.94 | 11.5 ± 1.42 |
| C18:3n-3 | 1.17 ± 0.01 | 2.65 ± 0.14 | 1.96 ± 0.05 | 1.36 ± 0.08 | 2.52 ± 0.35 |
| Σ PUFA | 11.28 ± 0.24 | 41.9 ± 1.00 | 33.2 ± 1.03 | 23.4 ± 1.03 | 14.0 ± 1.07 |
| n-6/n-3 | 8.69 ± 0.14 | 14.8 ± 0.48 | 15.9 ± 0.13 | 16.2 ± 0.27 | 4.66 ± 1.21 |
| Antioxidant concentration (mg/kg DM) | |||||
| α-tocopherol | 4.05 ± 0.62 | 251 ± 18.9 | 212 ± 14.1 | 213 ± 12.9 | 2.09 ± 0.85 |
| γ-tocopherol | 0.90 ± 0.17 | 7.44 ± 5.75 | 9.26 ± 5.34 | 11.7 ± 2.04 | 4.94 ± 6.01 |
| δ-tocopherol | 0.29 ± 0.02 | 1.58 ± 0.53 | 1.95 ± 0.34 | 1.80 ± 0.11 | 0.68 ± 0.77 |
| Total polyphenols (g tannic acid eq/kg DM) | 21.5 ± 0.28 | 8.00 ± 0.85 | 9.90 ± 0.86 | 12.1 ± 1.10 | 13.5 ± 1.40 |
| C0% | C15% | C30% | SEM 1 | p-Value | |
|---|---|---|---|---|---|
| Total FA | 1800 | 2138 | 2293 | 0.15 | 0.058 |
| C10:0 | 1.05 | 1.52 | 1.23 | 0.34 | 0.628 |
| C11:0 | 0.16 | 0.2 | 0.19 | 0.04 | 0.665 |
| C12:0 | 4.31 | 4.83 | 4.63 | 0.53 | 0.784 |
| C13:0 | 11.1 | 10.5 | 9.50 | 0.56 | 0.122 |
| C14:0 | 58.5 | 66.9 | 63 | 6.25 | 0.642 |
| C15:0 | 17.3 | 17.2 | 14.9 | 0.78 | 0.059 |
| C16:0 | 432 | 522 | 560 | 36.2 | 0.052 |
| C17:0 | 32.7 | 34.1 | 33.0 | 2.60 | 0.916 |
| C18:0 | 252 b | 303 ab | 330 a | 19.7 | 0.029 |
| C19:0 | 0.66 | 1.02 | 0.97 | 0.14 | 0.165 |
| C20:0 | 1.82 | 1.78 | 1.64 | 0.11 | 0.479 |
| C21:0 | 0.65 | 0.96 | 0.85 | 0.11 | 0.185 |
| C22:0 | 0.45 | 0.63 | 0.86 | 0.11 | 0.051 |
| C24:0 | 0.07 | 0.08 | 0.08 | 0.02 | 0.950 |
| ∑iso-OBCFA | 27.9 a | 28.1 a | 24.5 b | 1.10 | 0.041 |
| ∑anteiso-OBCFA | 13.09 | 13.12 | 11.06 | 0.76 | 0.096 |
| ∑OBCFA | 41.0 a | 41.2 a | 35.5 b | 1.78 | 0.047 |
| ∑DMA | 60.3 b | 72.6 ab | 91.5 a | 7.26 | 0.016 |
| ∑SFA Me | 11.5 | 12.8 | 13.3 | 0.91 | 0.418 |
| ∑SFA | 813 | 967 | 1022 | 63.8 | 0.077 |
| C0% | C15% | C30% | SEM 1 | p-Value | |
|---|---|---|---|---|---|
| C12:1 | 0.28 | 0.23 | 0.27 | 0.03 | 0.411 |
| C14:1 | 2.19 | 2.03 | 1.84 | 0.17 | 0.389 |
| C15:1 | 1.89 | 2.11 | 2.15 | 0.17 | 0.512 |
| C16:1 | 36.0 | 39.5 | 43.2 | 3.52 | 0.368 |
| cis-C16:1 | 32.3 | 35.7 | 39.4 | 3.50 | 0.332 |
| trans-C16:1 | 3.70 | 3.76 | 3.77 | 0.33 | 0.985 |
| C17:1 | 27.1 | 28.9 | 34.5 | 2.48 | 0.101 |
| cis-C17:1 | 25.6 | 27.4 | 33.3 | 2.59 | 0.083 |
| trans-C17:1 | 1.47 | 1.52 | 1.26 | 0.12 | 0.264 |
| C18:1 | 640 | 765 | 837 | 59.4 | 0.080 |
| cis9-C18:1 | 528 | 619 | 685 | 52.0 | 0.098 |
| cis11-C18:1 | 41.9 b | 51.7 ab | 63.6 a | 5.66 | 0.026 |
| trans10-C18:1 | 24.8 | 34.4 | 27.2 | 3.73 | 0.150 |
| trans11-C18:1 | 20.6 | 27.0 | 26.9 | 3.08 | 0.240 |
| trans10/11-C18:1 | 1.26 ab | 1.33 b | 1.01 a | 0.09 | 0.041 |
| C20:1 | 1.66 | 1.59 | 1.48 | 0.12 | 0.530 |
| C22:1 | 0.54 | 0.46 | 0.55 | 0.05 | 0.272 |
| C24:1 | 0.11 | 0.10 | 0.10 | 0.02 | 0.930 |
| ∑cis-MUFA | 654 | 767 | 857 | 61.5 | 0.086 |
| ∑trans-MUFA | 54.7 | 72.4 | 64.4 | 6.71 | 0.200 |
| ∑MUFA | 709 | 840 | 921 | 65.0 | 0.088 |
| C18:2 | 159 b | 191 ab | 200 a | 11.0 | 0.038 |
| C18:2n-6 | 153 b | 184 ab | 192 a | 10.4 | 0.039 |
| C19:2n-6 | 0.9 | 0.77 | 0.67 | 0.08 | 0.123 |
| C18:3n-6 | 1.41 | 1.54 | 1.53 | 0.10 | 0.620 |
| C18:3n-3 | 7.13 b | 8.41 ab | 9.42 a | 0.58 | 0.035 |
| C20:2n-6 | 1.43 | 1.39 | 1.33 | 0.09 | 0.744 |
| C20:3n-9 | 11.9 | 12.8 | 14.8 | 0.95 | 0.111 |
| C20:3n-6 | 5.32 | 5.67 | 5.82 | 0.26 | 0.397 |
| C20:3n-3 | 0.08 | 0.11 | 0.06 | 0.01 | 0.081 |
| C20:4n-6 ARA | 62.5 b | 72.5 ab | 77.7 a | 3.91 | 0.033 |
| C20:5n-3 EPA | 6.02 | 6.92 | 7.43 | 0.72 | 0.397 |
| C22:4n-6 | 4.79 | 5.10 | 5.07 | 0.26 | 0.653 |
| C22:5n-6 | 0.84 b | 1.27 ab | 1.37 a | 0.14 | 0.027 |
| C22:5n-3 DPA | 10.7 | 11.8 | 12.2 | 0.55 | 0.173 |
| C22:6n-3 DHA | 4.39 | 5.1 | 5.02 | 0.47 | 0.530 |
| ∑ CLA | 7.48 | 7.48 | 7.88 | 0.50 | 0.806 |
| cis9, trans11-C18:2 | 3.72 | 3.34 | 3.41 | 0.35 | 0.741 |
| ∑ n-6 | 230 b | 272 ab | 286 a | 14.3 | 0.030 |
| ∑ n-3 | 28.3 | 32.4 | 34.1 | 2.10 | 0.164 |
| ∑ PUFA | 284 b | 332 ab | 350 a | 16.8 | 0.028 |
| n-6/n-3 | 8.59 | 8.71 | 8.66 | 0.47 | 0.836 |
| PUFA/SFA | 0.36 | 0.35 | 0.35 | 0.37 | 0.842 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bottegal, D.N.; Lobón, S.; Latorre, M.Á.; Bertolín, J.R.; Álvarez-Rodríguez, J. Colour Stability, Fatty Acid Profile, and Lipid Oxidation in Meat Stored in Modified Atmosphere Packaging from Light Lambs Fed with Concentrate with Carob Pulp (Ceratonia siliqua L.). Antioxidants 2023, 12, 1482. https://doi.org/10.3390/antiox12081482
Bottegal DN, Lobón S, Latorre MÁ, Bertolín JR, Álvarez-Rodríguez J. Colour Stability, Fatty Acid Profile, and Lipid Oxidation in Meat Stored in Modified Atmosphere Packaging from Light Lambs Fed with Concentrate with Carob Pulp (Ceratonia siliqua L.). Antioxidants. 2023; 12(8):1482. https://doi.org/10.3390/antiox12081482
Chicago/Turabian StyleBottegal, Diego Nicolas, Sandra Lobón, María Ángeles Latorre, Juan Ramón Bertolín, and Javier Álvarez-Rodríguez. 2023. "Colour Stability, Fatty Acid Profile, and Lipid Oxidation in Meat Stored in Modified Atmosphere Packaging from Light Lambs Fed with Concentrate with Carob Pulp (Ceratonia siliqua L.)" Antioxidants 12, no. 8: 1482. https://doi.org/10.3390/antiox12081482
APA StyleBottegal, D. N., Lobón, S., Latorre, M. Á., Bertolín, J. R., & Álvarez-Rodríguez, J. (2023). Colour Stability, Fatty Acid Profile, and Lipid Oxidation in Meat Stored in Modified Atmosphere Packaging from Light Lambs Fed with Concentrate with Carob Pulp (Ceratonia siliqua L.). Antioxidants, 12(8), 1482. https://doi.org/10.3390/antiox12081482