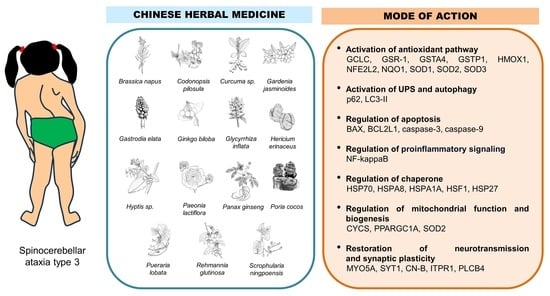
Oxidative Stress in Spinocerebellar Ataxia Type 3 and Its Attenuation by Herbal Remedies in Traditional Chinese Medicine: A Systematic Review
Abstract
:1. Introduction
2. The ATXN3 Gene and ATXN3 Protein
3. Oxidative Stress and Progression of SCA3
3.1. Inactivation of Forkhead Box O4 Transcription Factor
3.2. Inactivation of BCL2L1 Anti-Apoptotic Protein
3.3. Inactivation of NFE2L2 Transcription Factor
4. Involvement of Mitochondrial Dysfunction in the Progression of SCA3
5. Impairment of DNA Damage Response in the Progression of SCA3
6. Involvement of Antioxidant Defense Mechanisms in the Management of SCA3
7. Traditional Chinese Medicine as an Alternative in the Management of SCA3
8. Materials and Methods
8.1. Registration
8.2. Search Strategy
8.3. Eligibility Criteria
8.4. Data Extraction and Analysis
9. Results
10. Discussion
10.1. Brassica napus L.
10.2. Curcuma sp.
10.3. Gardenia jasminoides Ellis
10.4. Gastrodia elata Blume
10.5. Glycyrrhiza inflata Batalin
10.6. Hericium erinaceus (Bull.: Fr.) Pers.
10.7. Hyptis sp.
10.8. Paeonia lactiflora Pall.
10.9. Pueraria lobata (Willd.) Ohwi
10.10. Others
11. Limitations and Future Prospects
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zeng, S.; Zeng, J.; He, M.; Zeng, X.; Zhou, Y.; Liu, Z.; Jiang, H.; Tang, B.; Wang, J. Chinese homozygous Machado–Joseph disease (MJD)/SCA3: A case report. J. Hum. Genet. 2015, 60, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Jayadev, S.; Bird, T.D. Hereditary ataxias: Overview. Genet. Med. 2013, 15, 673–683. [Google Scholar] [CrossRef] [PubMed]
- van de Warrenburg, B.P.C.; Sinke, R.J.; Verschuuren-Bemelmans, C.C.; Scheffer, H.; Brunt, E.R.; Ippel, P.F.; Maat-Kievit, J.A.; Dooijes, D.; Notermans, N.C.; Lindhout, D.; et al. Spinocerebellar ataxias in the Netherlands: Prevalence and age at onset variance analysis. Neurology 2002, 58, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Ruano, L.; Melo, C.; Silva, M.C.; Coutinho, P. The global epidemiology of hereditary ataxia and spastic paraplegia: A systematic review of prevalence studies. Neuroepidemiology 2014, 42, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, P.; Ruano, L.; Loureiro, J.L.; Cruz, V.T.; Barros, J.; Tuna, A.; Barbot, C.; Guimarães, J.; Alonso, I.; Silveira, I.; et al. Hereditary ataxia and spastic paraplegia in Portugal: A population-based prevalence study. JAMA Neurol. 2013, 70, 746–755. [Google Scholar] [CrossRef]
- Erichsen, A.K.; Koht, J.; Stray-Pedersen, A.; Abdelnoor, M.; Tallaksen, C.M. Prevalence of hereditary ataxia and spastic paraplegia in southeast Norway: A population-based study. Brain 2009, 132, 1577–1588. [Google Scholar] [CrossRef]
- Tsuji, S.; Onodera, O.; Goto, J.; Nishizawa, M.; Study Group on Ataxic Diseases. Sporadic ataxias in Japan—A population-based epidemiological study. Cerebellum 2008, 7, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Calafell, F.; Gaspar, C.; Wong, V.C.; Silveira, I.; Nicholson, G.A.; Brunt, E.R.; Tranebjaerg, L.; Stevanin, G.; Hsieh, M.; et al. Asian origin for the worldwide-spread mutational event in Machado-Joseph disease. Arch. Neurol. 2007, 64, 1502–1508. [Google Scholar] [CrossRef]
- Bettencourt, C.; Santos, C.; Kay, T.; Vasconcelos, J.; Lima, M. Analysis of segregation patterns in Machado–Joseph disease pedigrees. J. Hum. Genet. 2008, 53, 920–923. [Google Scholar] [CrossRef]
- Klockgether, T.; Mariotti, C.; Paulson, H.L. Spinocerebellar ataxia. Nat. Rev. Dis. 2019, 5, 24. [Google Scholar] [CrossRef]
- Tan, S.; Wang, R.H.; Niu, H.X.; Shi, C.H.; Mao, C.Y.; Zhang, R.; Song, B.; Sun, S.L.; Liu, X.J.; Hou, H.M.; et al. Nerve growth factor for the treatment of spinocerebellar ataxia type 3: An open-label study. Chin. Med. J. 2015, 128, 291–294. [Google Scholar] [CrossRef]
- Rüb, U.; Schöls, L.; Paulson, H.; Auburger, G.; Kermer, P.; Jen, J.C.; Seidel, K.; Korf, H.W.; Deller, T. Clinical features, neurogenetics and neuropathology of the polyglutamine spinocerebellar ataxias type 1, 2, 3, 6 and 7. Prog. Neurobiol. 2013, 104, 38–66. [Google Scholar] [CrossRef]
- McLoughlin, H.S.; Moore, L.R.; Paulson, H.L. Pathogenesis of SCA3 and implications for other polyglutamine diseases. Neurobiol. Dis. 2020, 134, 104635. [Google Scholar] [CrossRef]
- Matos, C.A.; de Macedo-Ribeiro, S.; Carvalho, A.L. Polyglutamine diseases: The special case of ataxin-3 and Machado-Joseph disease. Prog. Neurobiol. 2011, 95, 26–48. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchelli, S.; De Palma, A.; Riva, M.; D’Urzo, A.; Pozzi, C.; Pastori, V.; Comelli, F.; Fusi, P.; Vanoni, M.; Tortora, P.; et al. Proteomic and biochemical analyses unveil tight interaction of ataxin-3 with tubulin. Int. J. Biochem. Cell Biol. 2009, 41, 2485–2492. [Google Scholar] [CrossRef]
- Rodrigues, A.J.; do Carmo Costa, M.; Silva, T.L.; Ferreira, D.; Bajanca, F.; Logarinho, E.; Maciel, P. Absence of ataxin-3 leads to cytoskeletal disorganization and increased cell death. Biochim. Biophys. Acta 2010, 1803, 1154–1163. [Google Scholar] [CrossRef]
- Harmuth, T.; Prell-Schicker, C.; Weber, J.J.; Gellerich, F.; Funke, C.; Drießen, S.; Magg, J.C.D.; Krebiehl, G.; Wolburg, H.; Hayer, S.N.; et al. Mitochondrial morphology, function and homeostasis are impaired by expression of an N-terminal calpain cleavage fragment of ataxin-3. Front. Mol. Neurosci. 2018, 11, 368. [Google Scholar] [CrossRef] [PubMed]
- do Carmo Costa, M.; Paulson, H.L. Toward understanding Machado-Joseph disease. Prog. Neurobiol. 2012, 97, 239–257. [Google Scholar] [CrossRef]
- Li, F.; Macfarlan, T.; Pittman, R.N.; Chakravarti, D. Ataxin-3 is a histone-binding protein with two independent transcriptional corepressor activities. J. Biol. Chem. 2002, 277, 45004–45012. [Google Scholar] [CrossRef]
- Evert, B.O.; Araujo, J.; Vieira-Saecker, A.M.; de Vos, R.A.; Harendza, S.; Klockgether, T.; Wüllner, U. Ataxin-3 represses transcription via chromatin binding, interaction with histone deacetylase 3, and histone deacetylation. J. Neurosci. 2006, 26, 11474–11486. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Saha, S.; Chakraborty, A.; Silva-Fernandes, A.; Mandal, S.M.; Neves-Carvalho, A.; Liu, Y.; Pandita, R.K.; Hegde, M.L.; Hegde, P.M.; et al. The role of the mammalian DNA end-processing enzyme polynucleotide kinase 3′-phosphatase in spinocerebellar ataxia type 3 pathogenesis. PLoS Genet. 2015, 11, e1004749. [Google Scholar] [CrossRef]
- Tu, Y.; Liu, H.; Zhu, X.; Shen, H.; Ma, X.; Wang, F.; Huang, M.; Gong, J.; Li, X.; Wang, Y.; et al. Ataxin-3 promotes genome integrity by stabilizing Chk1. Nucleic Acids Res. 2017, 45, 4532–4549. [Google Scholar] [CrossRef]
- Pfeiffer, A.; Luijsterburg, M.S.; Acs, K.; Wiegant, W.W.; Helfricht, A.; Herzog, L.K.; Minoia, M.; Böttcher, C.; Salomons, F.A.; van Attikum, H.; et al. Ataxin-3 consolidates the MDC1-dependent DNA double-strand break response by counteracting the SUMO-targeted ubiquitin ligase RNF4. EMBO J. 2017, 36, 1066–1083. [Google Scholar] [CrossRef]
- Matos, C.A.; Carmona, V.; Vijayakumar, U.G.; Lopes, S.; Albuquerque, P.; Conceição, M.; Nobre, R.J.; Nóbrega, C.; de Almeida, L.P. Gene therapies for polyglutamine diseases. Adv. Exp. Med. 2018, 1049, 395–438. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Okamoto, T.; Taniwaki, M.; Aizawa, M.; Inoue, M.; Katayama, S.; Kawakami, H.; Nakamura, S.; Nishimura, M.; Akiguchi, I. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat. Genet. 1994, 8, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Haberhausen, G.; Damian, M.S.; Leweke, F.; Müller, U. Spinocerebellar ataxia, type 3 (SCA3) is genetically identical to Machado-Joseph disease (MJD). J. Neurol. Sci. 1995, 132, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Paulson, H.L. Dominantly inherited ataxias: Lessons learned from Machado-Joseph disease/spinocerebellar ataxia type 3. Semin. Neurol. 2007, 27, 133–142. [Google Scholar] [CrossRef]
- Li, Q.F.; Cheng, H.L.; Yang, L.; Ma, Y.; Zhao, J.J.; Dong, Y.; Wu, Z.Y. Clinical features and genetic characteristics of homozygous spinocerebellar ataxia type 3. Mol. Genet. Genom. Med. 2020, 8, e1314. [Google Scholar] [CrossRef] [PubMed]
- Maciel, P.; Gaspar, C.; DeStefano, A.L.; Silveira, I.; Coutinho, P.; Radvany, J.; Dawson, D.M.; Sudarsky, L.; Guimarães, J.; Loureiro, J.E. Correlation between CAG repeat length and clinical features in Machado-Joseph disease. Am. J. Hum. Genet. 1995, 57, 54–61. [Google Scholar] [PubMed]
- Maruyama, H.; Nakamura, S.; Matsuyama, Z.; Sakai, T.; Doyu, M.; Sobue, G.; Seto, M.; Tsujihata, M.; Oh-I, T.; Nishio, T. Molecular features of the CAG repeats and clinical manifestation of Machado-Joseph disease. Hum. Mol. Genet. 1995, 4, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Dürr, A.; Stevanin, G.; Cancel, G.; Duyckaerts, C.; Abbas, N.; Didierjean, O.; Chneiweiss, H.; Benomar, A.; Lyon-Caen, O.; Julien, J.; et al. Spinocerebellar ataxia 3 and Machado-Joseph disease: Clinical, molecular, and neuropathological features. Ann. Neurol. 1996, 39, 490–499. [Google Scholar] [CrossRef]
- Yang, H.; Hu, H.Y. Sequestration of cellular interacting partners by protein aggregates: Implication in a loss-of-function pathology. FEBS J. 2016, 283, 3705–3717. [Google Scholar] [CrossRef]
- Gao, R.; Liu, Y.; Silva-Fernandes, A.; Fang, X.; Paulucci-Holthauzen, A.; Chatterjee, A.; Zhang, H.L.; Matsuura, T.; Choudhary, S.; Ashizawa, T.; et al. Inactivation of PNKP by mutant ATXN3 triggers apoptosis by activating the DNA damage-response pathway in SCA3. PLoS Genet. 2015, 11, e1004834. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Gkekas, I.; Gioran, A.; Boziki, M.K.; Grigoriadis, N.; Chondrogianni, N.; Petrakis, S. Oxidative stress and neurodegeneration: Interconnected processes in polyQ diseases. Antioxidants 2021, 10, 1450. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef] [PubMed]
- Seidel, K.; den Dunnen, W.F.; Schultz, C.; Paulson, H.; Frank, S.; de Vos, R.A.; Brunt, E.R.; Deller, T.; Kampinga, H.H.; Rüb, U. Axonal inclusions in spinocerebellar ataxia type 3. Acta Neuropathol. 2010, 120, 449–460. [Google Scholar] [CrossRef]
- Evers, M.M.; Toonen, L.J.A.; van Roon-Mom, W.M.C. Ataxin-3 protein and RNA toxicity in spinocerebellar ataxia type 3: Current insights and emerging therapeutic strategies. Mol. Neurobiol. 2014, 49, 1513–1531. [Google Scholar] [CrossRef]
- de Assis, A.M.; Saute, J.A.M.; Longoni, A.; Haas, C.B.; Torrez, V.R.; Brochier, A.W.; Souza, G.N.; Furtado, G.V.; Gheno, T.C.; Russo, A.; et al. Peripheral oxidative stress biomarkers in spinocerebellar ataxia type 3/Machado-Joseph disease. Front. Neurol. 2017, 8, 485. [Google Scholar] [CrossRef]
- Araujo, J.; Breuer, P.; Dieringer, S.; Krauss, S.; Dorn, S.; Zimmermann, K.; Pfeifer, A.; Klockgether, T.; Wuellner, U.; Evert, B.O. FOXO4-dependent upregulation of superoxide dismutase-2 in response to oxidative stress is impaired in spinocerebellar ataxia type 3. Hum. Mol. Genet. 2011, 20, 2928–2941. [Google Scholar] [CrossRef]
- Yu, Y.C.; Kuo, C.L.; Cheng, W.L.; Liu, C.S.; Hsieh, M. Decreased antioxidant enzyme activity and increased mitochondrial DNA damage in cellular models of Machado-Joseph disease. J. Neurosci Res. 2009, 87, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- van der Horst, A.; Burgering, B.M. Stressing the role of FoxO proteins in lifespan and disease. Nat. Rev. Mol. 2007, 8, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Li, X.; Zhu, X.; Liu, X.; Guo, C.; Jia, D.; Tang, T.S. Determining the fate of neurons in SCA3: ATX3, a rising decision maker in response to DNA stresses and beyond. Front. Cell Dev. Biol. 2020, 8, 619911. [Google Scholar] [CrossRef] [PubMed]
- Loo, L.S.W.; Soetedjo, A.P.; Lau, H.; Ng, N.H.J.; Ghosh, S.; Nguyen, L.; Krishnan, V.G.; Choi, H.; Roca, X.; Hoon, S.; et al. BCL-xL/BCL2L1 is a critical anti-apoptotic protein that promotes the survival of differentiating pancreatic cells from human pluripotent stem cells. Cell Death Dis. 2020, 11, 378. [Google Scholar] [CrossRef]
- Opferman, J.T.; Kothari, A. Anti-apoptotic BCL-2 family members in development. Cell Death Differ. 2018, 25, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vargas, M.P.A.; Chipuk, E. Physiological and pharmacological control of BAK, BAX, and beyond. Trends Cell Biol. 2016, 26, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, H.; Wang, P.; Ren, H.; Chen, D.; Ying, Z.; Wang, G. Ataxin-3 protects cells against H2O2-induced oxidative stress by enhancing the interaction between Bcl-X(L) and Bax. Neuroscience 2013, 243, 14–21. [Google Scholar] [CrossRef]
- Ming, L.; Wang, P.; Bank, A.; Yu, J.; Zhang, L. PUMA dissociates Bax and Bcl-X(L) to induce apoptosis in colon cancer cells. J. Biol. Chem. 2006, 281, 16034–16042. [Google Scholar] [CrossRef]
- Eno, C.O.; Zhao, G.; Olberding, K.E.; Li, C. The Bcl-2 proteins Noxa and Bcl-xL co-ordinately regulate oxidative stress-induced apoptosis. Biochem. J. 2012, 444, 69–78. [Google Scholar] [CrossRef]
- Chou, A.H.; Yeh, T.H.; Kuo, Y.L.; Kao, Y.C.; Jou, M.J.; Hsu, C.Y.; Tsai, S.R.; Kakizuka, A.; Wang, H.L. Polyglutamine-expanded ataxin-3 activates mitochondrial apoptotic pathway by upregulating Bax and downregulating Bcl-xL. Neurobiol. Dis. 2006, 21, 333–345. [Google Scholar] [CrossRef]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef]
- Tsai, H.F.; Tsai, H.J.; Hsieh, M. Full-length expanded ataxin-3 enhances mitochondrial-mediated cell death and decreases Bcl-2 expression in human neuroblastoma cells. Biochem. Biophys. Res. Commun. 2004, 324, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.H.; Chen, Y.L.; Chiu, C.C.; Yuan, S.J.; Weng, Y.H.; Yeh, T.H.; Lin, Y.L.; Fang, J.M.; Wang, H.L. T1-11 and JMF1907 ameliorate polyglutamine-expanded ataxin-3-induced neurodegeneration, transcriptional dysregulation and ataxic symptom in the SCA3 transgenic mouse. Neuropharmacology 2015, 99, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, P.; Unni, S.; Krishnappa, G.; Padmanabhan, B. The Keap1-Nrf2 pathway: Promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys. Rev. 2017, 9, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Chen, W.L.; Wu, Y.R.; Lin, T.H.; Wu, Y.C.; Chao, C.Y.; Lin, J.Y.; Lee, L.C.; Chen, Y.C.; Lee-Chen, G.J.; et al. Aqueous extract of Gardenia jasminoides targeting oxidative stress to reduce polyQ aggregation in cell models of spinocerebellar ataxia 3. Neuropharmacology 2014, 81, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Chang, J.C.; Lin, W.Y.; Li, C.C.; Hsieh, M.; Chen, H.W.; Wang, T.S.; Wu, W.T.; Liu, C.S.; Liu, K.L. Caffeic acid and resveratrol ameliorate cellular damage in cell and Drosophila models of spinocerebellar ataxia type 3 through upregulation of Nrf2 pathway. Free Radic. Biol. Med. 2018, 115, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.D.; Teixeira-Castro, A.; Maciel, P. From pathogenesis to novel therapeutics for spinocerebellar ataxia type 3: Evading potholes on the way to translation. Neurotherapeutics 2019, 16, 1009–1031. [Google Scholar] [CrossRef] [PubMed]
- Matos, C.A.; de Almeida, L.P.; Nóbrega, C. Machado-Joseph disease/spinocerebellar ataxia type 3: Lessons from disease pathogenesis and clues into therapy. J. Neurochem. 2019, 148, 8–28. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Todi, S.V.; Paulson, H.L.; do Carmo Costa, M. Regional and age-dependent changes in ubiquitination in cellular and mouse models of spinocerebellar ataxia type 3. Front. Mol. Neurosci. 2023, 16, 1154203. [Google Scholar] [CrossRef]
- Kazachkova, N.; Raposo, M.; Montiel, R.; Cymbron, T.; Bettencourt, C.; Silva-Fernandes, A.; Silva, S.; Maciel, P.; Lima, M. Patterns of mitochondrial DNA damage in blood and brain tissues of a transgenic mouse model of Machado-Joseph disease. Neurodegener. Dis. 2013, 11, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.Y.; Jhang, Y.L.; Cheng, P.H.; Chang, Y.F.; Mao, S.H.; Yang, H.I.; Lin, C.W.; Chen, C.M.; Yang, S.H. The truncated C-terminal fragment of mutant ATXN3 disrupts mitochondria dynamics in spinocerebellar ataxia type 3 models. Front. Mol. Neurosci. 2017, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.H.; Lin, A.C.; Hong, K.Y.; Hu, S.H.; Chen, Y.L. p53 activation mediates polyglutamine-expanded ataxin-3 upregulation of Bax expression in cerebellar and pontine nuclei neurons. Neurochem. Int. 2011, 58, 145–152. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Riveros, M.E.; Ávila, A.; Schruers, K.; Ezquer, F. Antioxidant biomolecules and their potential for the treatment of difficult-to-treat depression and conventional treatment-resistant depression. Antioxidants 2022, 11, 540. [Google Scholar] [CrossRef]
- Lopes-Ramos, C.M.; Pereira, T.C.; Dogini, D.B.; Gilioli, R.; Lopes-Cendes, I. Lithium carbonate and coenzyme Q10 reduce cell death in a cell model of Machado-Joseph disease. Braz. J. Med. Biol. Res. 2016, 49, e5805. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Chang, J.C.; Chao, Y.C.; Chan, H.; Hsieh, M.; Liu, C.S. In vitro efficacy and molecular mechanism of curcumin analog in pathological regulation of spinocerebellar ataxia type 3. Antioxidants 2022, 11, 1389. [Google Scholar] [CrossRef]
- Cornelius, N.; Wardman, J.H.; Hargreaves, I.P.; Neergheen, V.; Bie, A.S.; Tümer, Z.; Nielsen, J.E.; Nielsen, T.T. Evidence of oxidative stress and mitochondrial dysfunction in spinocerebellar ataxia type 2 (SCA2) patient fibroblasts: Effect of coenzyme Q10 supplementation on these parameters. Mitochondrion 2017, 34, 103–114. [Google Scholar] [CrossRef]
- Lo, R.Y.; Figueroa, K.P.; Pulst, S.M.; Lin, C.Y.; Perlman, S.; Wilmot, G.; Gomez, C.; Schmahmann, J.; Paulson, H.; Shakkottai, V.G.; et al. Coenzyme Q10 and spinocerebellar ataxias. Mov. Disord. 2015, 30, 214–220. [Google Scholar] [CrossRef]
- Niewiadomska-Cimicka, A.; Hache, A.; Trottier, Y. Gene deregulation and underlying mechanisms in spinocerebellar ataxias with polyglutamine expansion. Front. Neurosci. 2020, 14, 571. [Google Scholar] [CrossRef]
- Pacheco, L.S.; da Silveira, A.F.; Trott, A.; Houenou, L.J.; Algarve, T.D.; Belló, C.; da Cruz, I.B.M. Association between Machado–Joseph disease and oxidative stress biomarkers. Mutat. Res. Genet. Environ. Mutagen. 2013, 757, 99–103. [Google Scholar] [CrossRef]
- Se, A.T. Five phases (wuxing). In Encyclopaedia of the History of Science, Technology, and Medicine in Non-Western Cultures, 3rd ed.; Selin, H., Ed.; Springer: Dordrecht, The Netherlands, 2016; pp. 1899–1901. [Google Scholar] [CrossRef]
- Liu, L.F.; Song, J.X.; Lu, J.H.; Huang, Y.Y.; Zeng, Y.; Chen, L.L.; Durairajan, S.S.K.; Han, Q.B.; Li, M. Tianma Gouteng Yin, a traditional Chinese medicine decoction, exerts neuroprotective effects in animal and cellular models of Parkinson’s disease. Sci. Rep. 2015, 5, 16862. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, P.; Brand, E. A concise classification of bencao (materia medica). Chin. Med. 2018, 13, 18. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network pharmacology databases for traditional Chinese medicine: Review and assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef]
- Putteeraj, M.; Lim, W.; Teoh, S.L.; Yahaya, M. Flavonoids and its neuroprotective effects on brain ischemia and neurodegenerative diseases. Curr. Drug Targets 2018, 19, 1710–1720. [Google Scholar] [CrossRef]
- Lew, S.Y.; Lim, S.H.; Lim, L.W.; Wong, K.H. Neuroprotective effects of Hericium erinaceus (Bull.: Fr.) Pers. against high-dose corticosterone-induced oxidative stress in PC-12 cells. BMC Complement. Med. Ther. 2020, 20, 340. [Google Scholar] [CrossRef]
- John, P.A.; Wong, K.H.; Naidu, M.; Sabaratnam, V.; David, P. Combination effects of curcumin and aqueous extract of Lignosus rhinocerotis mycelium on neurite outgrowth stimulation activity in PC-12 cells. Nat. Prod. Commun. 2013, 8, 711–714. [Google Scholar] [CrossRef]
- Lew, S.Y.; Yow, Y.Y.; Lim, L.W.; Wong, K.H. Antioxidant-mediated protective role of Hericium erinaceus (Bull.: Fr.) Pers. against oxidative damage in fibroblasts from Friedreich’s ataxia patient. Food Sci. Technol. 2020, 40, 264–272. [Google Scholar] [CrossRef]
- Li, C.; Huang, J.; Cheng, Y.C.; Zhang, Y.W. Traditional Chinese medicine in depression treatment: From molecules to systems. Front. Pharmacol. 2020, 11, 586. [Google Scholar] [CrossRef]
- Tao, F.; Cai, Y.; Deng, C.; Chen, Z.; Shen, Y.; Sun, H. A narrative review on traditional Chinese medicine prescriptions and bioactive components in epilepsy treatment. Ann. Transl. Med. 2022, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guo, J.; Yin, H.; Yin, C.; Peng, Y. Traditional Chinese medicine for neonatal hypoxic-ischemic encephalopathy: A Bayesian network meta-analysis. J. Ethnopharmacol. 2023, 319, 117317. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wang, J.; Yu, J.; Tang, Q.; Liu, X.; Zhang, Y.; Cui, D.; Zhu, Y.; Mei, Y.; Wang, Y.; et al. Therapy of traumatic brain injury by modern agents and traditional Chinese medicine. Chin. Med. 2023, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, J.; Li, C.; Zheng, W.; He, J.; Wu, Z.; Tang, J. Application of natural antioxidants from traditional Chinese medicine in the treatment of spinal cord injury. Front. Pharmacol. 2022, 13, 976757. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov. Chinese Medicine WT for Spinocerebellar Ataxia Type 3. Identifier NCT05038306. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05038306 (accessed on 10 March 2024).
- Guo, F.; Liu, X.; Cai, H.; Le, W. Autophagy in neurodegenerative diseases: Pathogenesis and therapy. Brain Pathol. 2018, 28, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Paulino, R.; Nóbrega, C. Autophagy in spinocerebellar ataxia type 3: From pathogenesis to therapeutics. Int. J. Mol. Sci. 2023, 24, 7405. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021, 372, n71. [Google Scholar] [CrossRef]
- Ravikumar, B.; Vacher, C.; Berger, Z.; Davies, J.E.; Luo, S.; Oroz, L.G.; Rubinsztein, D.C. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat. Genet. 2004, 36, 585–595. [Google Scholar] [CrossRef]
- Seidel, K.; Meister, M.; Dugbartey, G.J.; Zijlstra, M.P.; Vinet, J.; Brunt, E.R.; van Leeuwen, F.W.; Rüb, U.; Kampinga, H.H.; den Dunnen, W.F. Cellular protein quality control and the evolution of aggregates in spinocerebellar ataxia type 3 (SCA3). Neuropathol. Appl. Neurobiol. 2012, 38, 548–558. [Google Scholar] [CrossRef]
- Pohl, F.; Teixeira-Castro, A.; Costa, M.D.; Lindsay, V.; Fiúza-Fernandes, J.; Goua, M.; Bermano, G.; Russell, W.; Maciel, P.; Lin, P.K.T. GST-4-dependent suppression of neurodegeneration in C. elegans models of Parkinson’s and Machado-Joseph disease by rapeseed pomace extract supplementation. Front. Neurosci. 2019, 13, 1091. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Weng, Y.T.; Chen, W.L.; Lin, T.H.; Chao, C.Y.; Lin, C.H.; Chen, I.C.; Lee, L.C.; Lin, H.Y.; Wu, Y.R.; et al. Aqueous extract of Glycyrrhiza inflata inhibits aggregation by upregulating PPARGC1A and NFE2L2-ARE pathways in cell models of spinocerebellar ataxia 3. Free Radic. Biol. Med. 2014, 71, 339–350. [Google Scholar] [CrossRef]
- Wu, Y.L.; Chen, S.C.; Chang, J.C.; Lin, W.Y.; Chen, C.C.; Li, C.C.; Hsieh, M.; Chen, H.W.; Chang, T.Y.; Liu, C.S.; et al. The protective effect of erinacine A-enriched Hericium erinaceus mycelium ethanol extract on oxidative stress-induced neurotoxicity in cell and Drosophila models of spinocerebellar ataxia type 3. Free Radic. Biol. Med. 2023, 195, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vilasboas-Campos, D.; Costa, M.D.; Teixeira-Castro, A.; Rios, R.; Silva, F.G.; Bessa, C.; Dias, A.C.P.; Maciel, P. Neurotherapeutic effect of Hyptis spp. leaf extracts in Caenorhabditis elegans models of tauopathy and polyglutamine disease: Role of the glutathione redox cycle. Free Radic. Biol. Med. 2021, 162, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Chen, W.L.; Lee, L.C.; Lin, C.H.; Kung, P.J.; Lin, T.H.; Wu, Y.C.; Wu, Y.R.; Chen, Y.C.; Lee-Chen, G.J.; et al. Aqueous extract of Paeonia lactiflora and paeoniflorin as aggregation reducers targeting chaperones in cell models of spinocerebellar ataxia 3. Evid. Based Complement. Alternat. Med. 2013, 2013, 471659. [Google Scholar] [CrossRef]
- Chen, I.C.; Chang, K.H.; Chen, Y.J.; Chen, Y.C.; Lee-Chen, G.J.; Chen, C.M. Pueraria lobata and daidzein reduce cytotoxicity by enhancing ubiquitin-proteasome system function in SCA3-iPSC-derived neurons. Oxid. Med. Cell Longev. 2019, 2019, 8130481. [Google Scholar] [CrossRef]
- Chen, I.C.; Chang, C.N.; Chen, W.L.; Lin, T.H.; Chao, C.Y.; Lin, C.H.; Lin, H.Y.; Cheng, M.L.; Chiang, M.C.; Lin, J.Y.; et al. Targeting ubiquitin proteasome pathway with traditional Chinese medicine for treatment of spinocerebellar ataxia type 3. Am. J. Chin. Med. 2019, 47, 63–95. [Google Scholar] [CrossRef]
- Pohl, F.; Lin, P.K.T. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: In vitro, in vivo and clinical trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef] [PubMed]
- Kortesniemi, M.; Vuorinen, A.L.; Sinkkonen, J.; Yang, B.; Rajala, A.; Kallio, H. NMR metabolomics of ripened and developing oilseed rape (Brassica napus) and turnip rape (Brassica rapa). Food Chem. 2015, 172, 63–70. [Google Scholar] [CrossRef]
- Kasprzak, M.; Houdijk, J.; Kightley, S.; Olukosi, O.; White, G.; Carre, P.; Wiseman, J. Effects of rapeseed variety and oil extraction method on the content and ileal digestibility of crude protein and amino acids in rapeseed cake and softly processed rapeseed meal fed to broiler chickens. Anim. Feed Sci. Technol. 2016, 213, 90–98. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Tułodziecka, A. Antioxidant capacity of rapeseed extracts obtained by conventional and ultrasound-assisted extraction. J. Am. Oil. Chem. Soc. 2014, 91, 2011–2019. [Google Scholar] [CrossRef]
- Sun, M.; Gao, Y.; Guo, C.; Cao, F.; Song, Z.; Xi Zhai, G. Enhancement of transport of curcumin to brain in mice by poly(n-butylcyanoacrylate) nanoparticle. J. Nanopart Res. 2010, 12, 3111–3122. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, D.K.; Dash, D.; Singh, R. Intranasal curcumin protects against LPS-induced airway remodeling by modulating toll-like receptor-4 (TLR-4) and matrixmetalloproteinase-9 (MMP-9) expression via affecting MAP kinases in mouse model. Inflammopharmacology 2019, 27, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Chaturvedi, M.; Mishra, S.; Kumar, P.; Somvanshi, P.; Chaturvedi, R. Reductive metabolites of curcumin and their therapeutic effects. Heliyon 2020, 6, e05469. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, M.; Yang, Z.; Tao, W.; Wang, P.; Tian, X.; Li, X.; Wang, W. Gardenia jasminoides Ellis: Ethnopharmacology, phytochemistry, and pharmacological and industrial applications of an important traditional Chinese medicine. J. Ethnopharmacol. 2020, 257, 112829. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.J.; Lim, K.H.; Jung, H.J.; Park, E.H. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J. Ethnopharmacol. 2006, 103, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Zhang, H.; Xue, W.; Ren, L.; Xia, B.; Zhou, X.; Wu, H.; Duan, J.; Chen, G. Optimization of supercritical fluid extraction of oil from the fruit of Gardenia jasminoides and its antidepressant activity. Molecules 2014, 19, 19350–19360. [Google Scholar] [CrossRef]
- Yin, F.; Liu, J.H. Research and application progress of Gardenia jasminoides. Chin. Herb. Med. 2018, 10, 362–370. [Google Scholar] [CrossRef]
- Wu, S.Y.; Wang, G.F.; Liu, Z.Q.; Rao, J.J.; Lü, L.; Xu, W.; Wu, S.G.; Zhang, J.J. Effect of geniposide, a hypoglycemic glucoside, on hepatic regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Acta Pharmacol. Sin. 2009, 30, 202–208. [Google Scholar] [CrossRef]
- Yin, F.; Liu, J.; Zheng, X.; Guo, L.; Xiao, H. Geniposide induces the expression of heme oxygenase-1 via PI3K/Nrf2-signaling to enhance the antioxidant capacity in primary hippocampal neurons. Biol. Pharm. Bull. 2010, 33, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, J.; Peng, M.; Meng, H.; Ma, H.; Cai, P.; Xu, Y.; Zhao, Q.; Si, G. A review on central nervous system effects of gastrodin. Front. Pharmacol. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.D.; Zhou, H.Y.; Sui, Y.P.; Du, X.L.; Wang, W.H.; Dai, L.; Sui, F.; Huo, H.R.; Jiang, T.L. The rhizome of Gastrodia elata Blume—An ethnopharmacological review. J. Ethnopharmacol. 2016, 189, 361–385. [Google Scholar] [CrossRef]
- Tan, W.; Zheng, Q.; Feng, K.; Feng, X.; Zhong, W.; Liao, C.; Li, S.; Liu, Y.; Hu, W. Neuroprotection of Gastrodia elata polyphenols against H2O2-induced PC12 cell cytotoxicity by reducing oxidative stress. Front. Pharmacol. 2022, 13, 1050775. [Google Scholar] [CrossRef]
- Bulpitt, C.J.; Li, Y.; Bulpitt, P.F.; Wang, J. The use of orchids in Chinese medicine. J. R. Soc. Med. 2007, 100, 558–563. [Google Scholar] [CrossRef]
- Sucher, N.J. Insights from molecular investigations of traditional Chinese herbal stroke medicines: Implications for neuroprotective epilepsy therapy. Epilepsy Behav. 2006, 8, 350–362. [Google Scholar] [CrossRef]
- Schachter, S.C. Botanicals and herbs: A traditional approach to treating epilepsy. Neurotherapeutics 2009, 6, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Heese, K. Gastrodia elata Blume (Tianma): Hope for brain aging and dementia. Evid. Based Complement. Alternat. Med. 2020, 2020, 8870148. [Google Scholar] [CrossRef]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Naidu, M.; David, P.; Abdulla, M.A.; Abdullah, N.; Kuppusamy, U.R.; Sabaratnam, V. Peripheral nerve regeneration following crush injury to rat peroneal nerve by aqueous extract of medicinal mushroom Hericium erinaceus (Bull.: Fr) Pers. (Aphyllophoromycetideae). Evid. Based Complement. Alternat. Med. 2011, 2011, 580752. [Google Scholar] [CrossRef] [PubMed]
- Samberkar, S.; Gandhi, S.; Naidu, M.; Wong, K.H.; Raman, J.; Sabaratnam, V. Lion’s mane, Hericium erinaceus and tiger milk, Lignosus rhinocerotis (higher basidiomycetes) medicinal mushrooms stimulate neurite outgrowth in dissociated cells of brain, spinal cord, and retina: An in vitro study. Int. J. Med. Mushrooms 2015, 17, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Ng, C.C.; Kanagasabapathy, G.; Yow, Y.Y.; Sabaratnam, V. An overview of culinary and medicinal mushrooms in neurodegeneration and neurotrauma research. Int. J. Med. Mushrooms 2017, 19, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Kim, H.G.; Kim, J.Y.; Kim, S.Y.; Cho, K.O. Hericium erinaceus extract reduces anxiety and depressive behaviors by promoting hippocampal neurogenesis in the adult mouse brain. J. Med. Food. 2018, 21, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.S.; Poon, C.H.; Roy, J.; Tsui, K.C.; Lew, S.Y.; Phang, M.W.L.; Tan, R.J.Y.; Cheng, P.G.; Fung, M.L.; Wong, K.H.; et al. Neurogenesis-dependent antidepressant-like activity of Hericium erinaceus in an animal model of depression. Chin. Med. 2021, 16, 132. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.S.; Khairuddin, S.; Tse, A.C.K.; Hiew, L.F.; Lau, C.L.; Tipoe, G.L.; Fung, M.L.; Wong, K.H.; Lim, L.W. Hericium erinaceus potentially rescues behavioural motor deficits through ERK-CREB-PSD95 neuroprotective mechanisms in rat model of 3-acetylpyridine-induced cerebellar ataxia. Sci. Rep. 2020, 10, 14945. [Google Scholar] [CrossRef] [PubMed]
- Lew, S.Y.; Phang, M.W.L.; Chong, P.S.; Roy, J.; Poon, C.H.; Yu, W.S.; Lim, L.W.; Wong, K.H. Discovery of therapeutics targeting oxidative stress in autosomal recessive cerebellar ataxia: A systematic review. Pharmaceuticals 2022, 15, 764. [Google Scholar] [CrossRef] [PubMed]
- Chau, S.C.; Chong, P.S.; Jin, H.; Tsui, K.C.; Khairuddin, S.; Tse, A.C.K.; Lew, S.Y.; Tipoe, G.L.; Lee, C.W.; Fung, M.L.; et al. Hericium erinaceus promotes anti-inflammatory effects and regulation of metabolites in an animal model of cerebellar ataxia. Int. J. Mol. 2023, 24, 6089. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.F.; Tung, S.Y.; Teng, C.C.; Shen, C.H.; Hsieh, M.C.; Huang, C.Y.; Lee, K.C.; Lee, L.Y.; Chen, W.P.; Chen, C.C.; et al. Post-treatment with erinacine A, a derived diterpenoid of H. erinaceus, attenuates neurotoxicity in MPTP model of Parkinson’s disease. Antioxidants 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Salinaro, A.T.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Cuzzocrea, S.; Di Paola, R.; Fusco, R.; et al. Key mechanisms and potential implications of Hericium erinaceus in NLRP3 inflammasome activation by reactive oxygen species during Alzheimer’s disease. Antioxidants 2021, 10, 1664. [Google Scholar] [CrossRef] [PubMed]
- Yanshree; Yu, W.S.; Fung, M.L.; Lee, C.W.; Lim, L.W.; Wong, K.H. The monkey head mushroom and memory enhancement in Alzheimer’s disease. Cells 2022, 11, 2284. [Google Scholar] [CrossRef]
- D’Amico, R.; Trovato Salinaro, A.; Fusco, R.; Cordaro, M.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Lo Dico, G.; Cuzzocrea, S.; Di Paola, R.; et al. Hericium erinaceus and Coriolus versicolor modulate molecular and biochemical changes after traumatic brain injury. Antioxidants 2021, 10, 898. [Google Scholar] [CrossRef]
- Kawagishi, H.; Ando, M.; Mizuno, T. Hericenone A and B as cytotoxic principles from the mushroom. Tetrahedron Lett. 1990, 31, 373–376. [Google Scholar] [CrossRef]
- Ma, B.J.; Shen, J.W.; Yu, H.Y.; Ruan, Y.; Wu, T.T.; Zhao, X. Hericenones and erinacines: Stimulators of nerve growth factor (NGF) biosynthesis in Hericium erinaceus. Mycology 2010, 1, 92–98. [Google Scholar] [CrossRef]
- Kawagishi, H.; Shirai, R.; Sakamoto, H.; Yoshida, S.; Ojima, F.; Ishiguro, Y. Erinapyrones A and B from the cultured mycelia of Hericium erinaceum. Chem. Lett. 1992, 21, 2475–2476. [Google Scholar] [CrossRef]
- Arnone, A.; Cardillo, R.; Nasini, G.; de Pava, O.V. Secondary mold metabolites: Part 46. Hericenes A-C and erinapyrone C, new metabolites produced by the fungus Hericium erinaceus. J. Nat. Prod. 1994, 57, 602–606. [Google Scholar] [CrossRef]
- Lu, Q.Q.; Tian, J.M.; Wei, J.; Gao, J.M. Bioactive metabolites from the mycelia of the basidiomycete Hericium erinaceum. Nat. Prod. Res. 2014, 28, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- Jesus, N.Z.; Falcão, H.S.; Lim, G.R.; Caldas Filho, M.R.; Sales, I.R.; Gomes, I.F.; Santos, S.G.; Tavares, J.F.; Barbosa-Filho, J.M.; Batista, L.M. Hyptis suaveolens (L.) Poit (Lamiaceae), a medicinal plant protects the stomach against several gastric ulcer models. J. Ethnopharmacol. 2013, 150, 982–988. [Google Scholar] [CrossRef]
- Paixão, M.S.; Melo, M.S.; Oliveira, M.G.; Santana, M.T.; Lima, A.C.; Damascena, N.P.; Dias, A.S.; Araujo, B.S.; Estevam, C.S.; Botelho, M.A.; et al. Hyptis pectinata: Redox protection and orofacial antinociception. Phytother. Res. 2013, 27, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, R.; Branquinho, N.; Hara, A.; Silva, F.; Kellner Filho, L.; Silva, M.; Januario, A.H. Effect of salicylic acid and silver nitrate on rutin production by Hyptis marrubioides cultured in vitro. Ciênc. Rural 2019, 49, e20180278. [Google Scholar] [CrossRef]
- Aremu, H.K.; Adekale, I.A.; Azeez, L.A.; Busari, H.K.; Adebisi, O.; Iwalewa, O.; Musa, D.A. Assessment of larvicidal and genotoxic potentials of extracts of Hyptis suaveolens against Culex quinquefasciatus based on enzyme profile and RAPD-PCR assay. Acta Trop. 2022, 229, 106384. [Google Scholar] [CrossRef]
- Raymundo, L.J.; Guilhon, C.C.; Alviano, D.S.; Matheus, M.E.; Antoniolli, A.R.; Cavalcanti, S.C.; Alves, P.B.; Alviano, C.S.; Fernandes, P.D. Characterisation of the anti-inflammatory and antinociceptive activities of the Hyptis pectinata (L.) Poit essential oil. J. Ethnopharmacol. 2011, 134, 725–732. [Google Scholar] [CrossRef]
- Mishra, P.; Sohrab, S.; Mishra, S.K. A review on the phytochemical and pharmacological properties of Hyptis suaveolens (L.) Poit. Futur. J. Pharm. Sci. 2021, 7, 65. [Google Scholar] [CrossRef]
- Tan, Y.Q.; Chen, H.W.; Li, J.; Wu, Q. Efficacy, chemical constituents, and pharmacological actions of Radix Paeoniae Rubra and Radix Paeoniae alba. Front. Pharmacol. 2020, 11, 1054. [Google Scholar] [CrossRef]
- Bae, T.; Jang, J.; Lee, H.; Song, J.; Chae, S.; Park, M.; Son, C.G.; Yoon, S.; Yoon, Y. Paeonia lactiflora root extract suppresses cancer cachexia by down-regulating muscular NF-κB signalling and muscle-specific E3 ubiquitin ligases in cancer-bearing mice. J. Ethnopharmacol. 2020, 246, 112222. [Google Scholar] [CrossRef]
- Ou, T.T.; Wu, C.H.; Hsu, J.D.; Chyau, C.C.; Lee, H.J.; Wang, C.J. Paeonia lactiflora Pall inhibits bladder cancer growth involving phosphorylation of Chk2 in vitro and in vivo. J. Ethnopharmacol. 2011, 135, 162–172. [Google Scholar] [CrossRef] [PubMed]
- He, D.Y.; Dai, S.M. Anti-inflammatory and immunomodulatory effects of Paeonia lactiflora Pall., a traditional Chinese herbal medicine. Front. Pharmacol. 2011, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, Y.C.; Lee, D.Y. Applications of Pueraria lobata in treating diabetics and reducing alcohol drinking. Chin. Herb. Med. 2019, 11, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wu, P.; Cai, Z.; Fang, Y.; Zhou, H.; Lasanajak, Y.; Tang, L.; Ye, L.; Hou, C.; Zhao, J. Puerariae lobatae Radix with chuanxiong Rhizoma for treatment of cerebral ischemic stroke by remodeling gut microbiota to regulate the brain-gut barriers. J. Nutr. Biochem. 2019, 65, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lam, T.N.; Zuo, Z. Radix Puerariae: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin Pharmacol. 2013, 53, 787–811. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Hong, S.S.; Shin, Y.S.; Hwang, B.Y.; Park, S.Y.; Lee, D. Phenolic compounds from Pueraria lobata protect PC12 cells against Aβ-induced toxicity. Arch. Pharm. Res. 2010, 33, 1651–1654. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Yuan, R.; Chen, X.; Xin, Q.; Wang, Y.; Shang, X.; Cong, W.; Chen, K. Puerarin reduces blood pressure in spontaneously hypertensive rats by targeting eNOS. Am. J. Chin. Med. 2019, 47, 19–38. [Google Scholar] [CrossRef]
- Wang, S.; Yao, W.; Zhu, X.; Wang, J.; Lu, L.; Zhu, N.; Lan, T.; Kuang, Y.; Zhu, W.; Liu, R.; et al. Exploring the mechanism of the antithrombotic effects of Pueraria lobata and Pueraria lobata var. thomsonii based on network pharmacology. J. Ethnopharmacol. 2023, 300, 115701. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.L.; Schaaf Teixeira, S.; Monteiro, M.H.; De-Oliveira, A.C.; Paumgartten, F.J.R. Traditional use and safety of herbal medicines. Rev. Bras. Farmacogn. 2014, 24, 248–257. [Google Scholar] [CrossRef]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Jütte, R.; Heinrich, M.; Helmstädter, A.; Langhorst, J.; Meng, G.; Niebling, W.; Pommerening, T.; Trampisch, H.J. Herbal medicinal products—Evidence and tradition from a historical perspective. J. Ethnopharmacol. 2017, 207, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Giardina, C.; Cutroneo, P.M.; Mocciaro, E.; Russo, G.T.; Mandraffino, G.; Basile, G.; Rapisarda, F.; Ferrara, R.; Spina, E.; Arcoraci, V. Adverse drug reactions in hospitalized patients: Results of the FORWARD (Facilitation of Reporting in Hospital Ward) study. Front. Pharmacol. 2018, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Che, C.T.; Wang, Z.J.; Chow, M.S.; Lam, C.W. Herb-herb combination for therapeutic enhancement and advancement: Theory, practice and future perspectives. Molecules 2013, 18, 5125–5141. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.Y.; Huang, W.T.; Lin, Y.C.; Hung, H.H.; Ou, S.C.; Chang, C.W.; Lin, H.E.; Lin, T.Y.; Chang, C.W.; Hung, H.C.; et al. Prescription system to calculate precise doses of Chinese herbal medicine to avoid toxic effects. Heliyon 2023, 9, e16612. [Google Scholar] [CrossRef]
- Su, X.; Yao, Z.; Li, S.; Sun, H. Synergism of Chinese herbal medicine: Illustrated by Danshen compound. Evid. Based Complement. Alternat. Med. 2016, 2016, 7279361. [Google Scholar] [CrossRef]
- Phang, M.W.L.; Lew, S.Y.; Chung, I.; Lim, W.K.; Lim, L.W.; Wong, K.H. Therapeutic roles of natural remedies in combating hereditary ataxia: A systematic review. Chin. Med. 2021, 16, 15. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; The International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Harkey, M.R.; Henderson, G.L. Effects of intrinsic fluorescence and quenching on fluorescence-based screening of natural products. Phytomedicine 2002, 9, 263–267. [Google Scholar] [CrossRef]
- Zhu, J.; van de Ven, W.J.; Verbiest, T.; Koeckelberghs, G.; Chen, C.; Cui, Y.; Vermorken, A.J. Polyphenols can inhibit furin in vitro as a result of the reactivity of their auto-oxidation products to proteins. Curr. Med. Chem. 2013, 20, 840–850. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Gkika, D.A.; Tolkou, A.K.; Lambropoulou, D.A.; Bikiaris, D.N.; Kokkinos, P.; Kalavrouziotis, I.K.; Kyzas, G.Z. Application of molecularly imprinted polymers (MIPs) as environmental separation tools. RSC Appl. Polym. 2024. [Google Scholar] [CrossRef]
- Canter, P.H.; Thomas, H.; Ernst, E. Bringing medicinal plants into cultivation: Opportunities and challenges for biotechnology. Trends Biotechnol. 2005, 23, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Ritthaphai, A.; Wattanapanitch, M.; Pithukpakorn, M.; Heepchantree, W.; Soi-Ampornkul, R.; Mahaisavariya, P.; Triwongwaranat, D.; Pattanapanyasat, K.; Vatanashevanopakorn, C. Derivation of an induced pluripotent stem cell line (MUSIi004-A) from dermal fibroblasts of a 48-year-old spinocerebellar ataxia type 3 patient. Stem Cell Res. 2018, 30, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Perdomini, M.; Hick, A.; Puccio, H.; Pook, M.A. Animal and cellular models of Friedreich ataxia. J. Neurochem. 2013, 126, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Narukawa, M. Randomized controlled trial data for successful new drug application for rare diseases in the United States. Orphanet J. Rare Dis. 2023, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Ibrahim, N.; Lau, Y.H.; Ariffin, N.; Md Desa, S.H.; Azizan, E.; Chin, L.K.; Md Rani, S.A.; Yakob, Y.; Datuk Puvanarajah, S.; van de Warrenburg, B. Frequency of spinocerebellar ataxia type 1, 2, 3,6 and 7 and clinical profile of spinocerebellar ataxia type 3 in Malaysia. Cerebellum Ataxias 2020, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Zesiewicz, T.A.; Greenstein, P.E.; Sullivan, K.L.; Wecker, L.; Miller, A.; Jahan, I.; Chen, R.; Perlman, S.L. A randomized trial of varenicline (chantix) for the treatment of spinocerebellar ataxia type 3. Neurology 2012, 78, 545–550. [Google Scholar] [CrossRef]
- Thomas, K.H.; Martin, R.M.; Knipe, D.W.; Higgins, J.P.; Gunnell, D. Risk of neuropsychiatric adverse events associated with varenicline: Systematic review and meta-analysis. BMJ 2015, 350, h1109. [Google Scholar] [CrossRef]
- Neves-Carvalho, A.; Logarinho, E.; Freitas, A.; Duarte-Silva, S.; do Carmo Costa, M.; Silva-Fernandes, A.; Martins, M.; Serra, S.C.; Lopes, A.T.; Paulson, H.L.; et al. Dominant negative effect of polyglutamine expansion perturbs normal function of ataxin-3 in neuronal cells. Hum. Mol. Genet. 2015, 24, 100–117. [Google Scholar] [CrossRef]
- Nóbrega, C.; Codêsso, J.M.; Mendonça, L.; Pereira de Almeida, L. RNA interference therapy for Machado-Joseph disease: Long-term safety profile of lentiviral vectors encoding short hairpin RNAs targeting mutant ataxin-3. Hum. Gene Ther. 2019, 30, 841–854. [Google Scholar] [CrossRef]
- Baum, C.; Düllmann, J.; Li, Z.; Fehse, B.; Meyer, J.; Williams, D.A.; von Kalle, C. Side effects of retroviral gene transfer into hematopoietic stem cells. Blood 2003, 101, 2099–2114. [Google Scholar] [CrossRef]
- Jakobsson, J.; Lundberg, C. Lentiviral vectors for use in the central nervous system. Mol. Ther. 2006, 13, 484–493. [Google Scholar] [CrossRef]
- McGarrity, G.J.; Hoyah, G.; Winemiller, A.; Andre, K.; Stein, D.; Blick, G.; Greenberg, R.N.; Kinder, C.; Zolopa, A.; Binder-Scholl, G.; et al. Patient monitoring and follow-up in lentiviral clinical trials. J. Gene Med. 2013, 15, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Ashizawa, T.; Kuo, S.H. Collaborative efforts for spinocerebellar ataxia research in the United States: CRC-SCA and READISCA. Front. Neurol. 2020, 11, 902. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Xie, Y.; Xiong, Z.; Yang, Y.; Xian, Y.; Ou, Z.; Song, B.; Chen, Y.; Xie, Y.; Li, H.; et al. CRISPR/Cas9-targeted deletion of polyglutamine in spinocerebellar ataxia type 3-derived induced pluripotent stem cells. Stem Cells Dev. 2018, 27, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ma, X.; Gao, F.; Guo, Y. Off-target effects in CRISPR/Cas9 gene editing. Front. Bioeng. Biotechnol. 2023, 11, 1143157. [Google Scholar] [CrossRef]






| Herbal Remedy | Model | Dose | Finding | Mode of Action | Ref. |
|---|---|---|---|---|---|
| Brassica napus L. | Transgenic C. elegans expressing mATXN3 |
|
|
| [92] |
| Curcuma sp. | SK-N-SH-MJD78 cells | 0.3–5 µM of JM17 |
|
| [68] |
| Gardenia jasminoides Ellis |
|
|
|
| [56] |
| Gastrodia elata Blume | Transgenic mice expressing mATXN3-Q79HA |
|
|
| [54] |
| Glycyrrhiza inflata Batalin |
|
|
|
| [93] |
| Hericium erinaceus (Bull.: Fr.) Pers. |
|
|
|
| [94] |
|
|
| |||
| Hyptis sp. |
|
|
|
| [95] |
|
|
| |||
| Paeonia lactiflora Pall. |
|
|
|
| [96] |
|
| ||||
| Pueraria lobata (Willd.) Ohwi |
|
|
|
| [97] |
Other
|
|
|
|
| [98] |
|
| ||||
|
|
|
| ||
|
|
|
|
| |
|
|
| |||
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Hisam, N.S.; Wong, K.H. Oxidative Stress in Spinocerebellar Ataxia Type 3 and Its Attenuation by Herbal Remedies in Traditional Chinese Medicine: A Systematic Review. Antioxidants 2024, 13, 375. https://doi.org/10.3390/antiox13030375
Mohd Hisam NS, Wong KH. Oxidative Stress in Spinocerebellar Ataxia Type 3 and Its Attenuation by Herbal Remedies in Traditional Chinese Medicine: A Systematic Review. Antioxidants. 2024; 13(3):375. https://doi.org/10.3390/antiox13030375
Chicago/Turabian StyleMohd Hisam, Nur Shahirah, and Kah Hui Wong. 2024. "Oxidative Stress in Spinocerebellar Ataxia Type 3 and Its Attenuation by Herbal Remedies in Traditional Chinese Medicine: A Systematic Review" Antioxidants 13, no. 3: 375. https://doi.org/10.3390/antiox13030375
APA StyleMohd Hisam, N. S., & Wong, K. H. (2024). Oxidative Stress in Spinocerebellar Ataxia Type 3 and Its Attenuation by Herbal Remedies in Traditional Chinese Medicine: A Systematic Review. Antioxidants, 13(3), 375. https://doi.org/10.3390/antiox13030375