Chlorophyll Oxidative Metabolism During the Phototrophic and Heterotrophic Growth of Scenedesmus obliquus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Media
2.2. Cultivation Conditions
2.3. Kinetic Parameters
2.4. Extraction of Photosynthetic Pigments
2.5. Identification of Photosynthetic Pigments by HPLC-ESI/APCI-HRTOF-MSn
2.6. Quantification of Photosynthetic Pigments by HPLC-UV-Visible Detection
2.7. Statistical Analysis
3. Results
3.1. Microalgae Growth/Kinetic Parameters
3.2. Pigment Profile During Phototrophic Growth
3.3. Pigment Evolution During Heterotrophic Growth
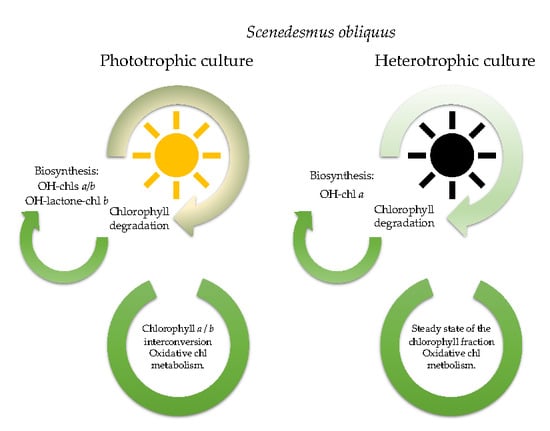
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Viera, I.; Roca, M.; Perez-Galvez, A. Mass Spectrometry of Non-allomerized Chlorophylls a and b Derivatives from Plants. Curr. Org. Chem. 2018, 22, 842–876. [Google Scholar] [CrossRef]
- Catarina, M.M.; Duarte, F.; Malcata, X. Supercritical fluid extraction of carotenoids and chlorophylls a, b and c, from a wild strain of Scenedesmus obliquus for use in food processing. J. Food Eng. 2013, 116, 478–482. [Google Scholar]
- Chacon-Lee, T.L.; González-Marino, G.E. Microalgae for “Healthy” Foods—Possibilities and Challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Green Natural Colorants. Molecules 2019, 24, 154. [Google Scholar] [CrossRef]
- Queiroz Zepka, L.; Jacob-Lopes, E.; Roca, M. Catabolism and bioactive properties of chlorophylls. Curr. Opin. Food Sci. 2019, 26, 94–100. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Böhm, V.; Courtney, P.D.; Schwartz, S.J. Antioxidant and antimutagenic activity of dietary chlorophyll derivatives determined by radical scavenging and bacterial reverse mutagenesis assays. Food Chem. Toxicol. 2002, 67, 2589–2595. [Google Scholar] [CrossRef]
- Kang, Y.R.; Park, J.; Jung, S.K.; Chang, Y.H. Synthesis, characterization, and functional properties of chlorophylls, pheophytins, and Zn-pheophytins. Food Chem. 2018, 245, 943–950. [Google Scholar] [CrossRef]
- Hoshina, C.; Tomita, K.; Shioi, Y. Antioxidant Activity of Chlorophylls: Its Structure-Activity Relationship. In Photosynthesis: Mechanisms and Effects; Garab, G., Ed.; Springer: Berlin, Germany, 1998; pp. 3281–3284. [Google Scholar]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Xavier, A.A.; Pérez-Gálvez, A. Carotenoids as a Source of Antioxidants in the Diet. Subcell. Biochem. 2016, 79, 359–375. [Google Scholar] [CrossRef]
- Chen, W.; Hsu, Y.; Chang, J.; Ho, S.; Wang, L.; We, Y. Enhancing production of lutein by a mixotrophic cultivation system using microalga Scenedesmus obliquus CWL-1. Bioresour. Technol. 2019, 291, 121891. [Google Scholar] [CrossRef]
- Ferreira, V.S.; Sant’Anna, C. Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World J. Microbiol. Biotechnol. 2017, 33, 20. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Nagarajan, D.; Zhanga, Q.; Chang, J.; Lee, D. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnol. Adv. 2018, 36, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.A. Greening in the dark: Light-independent chlorophyll biosynthesis from anoxygenic photosynthetic bacteria to gymnosperms. J. Photochem. Photobiol. B Biol. 1998, 43, 87–100. [Google Scholar] [CrossRef]
- Abeliovich, A.; Weisman, D. Role of heterotrophic nutrition in growth of the alga Scenedesmus obliquus in high-rate oxidation ponds. Appl. Environ. Microbiol. 1978, 35, 32–37. [Google Scholar] [PubMed]
- Van Wagenen, J.; De Francisci, D.; Angelidaki, I. Comparison of mixotrophic to cyclic autotrophic/heterotrophic growth strategies to optimize productivity of Chlorella sorokiniana. J. Appl. Phycol. 2015, 27, 1775–1782. [Google Scholar] [CrossRef]
- Sun, X.; Ren, L.; Zhao, Q.; Ji, X.; Huang, H. Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Flórez-Miranda, L.; Cañizares-Villanueva, O.; Melchy-Antonio, O.; Martínez-Jerónimo, F.; Mateo Flores-Ortíz, C. Two stage heterotrophy/photoinduction culture of Scenedesmus incrassatulus: Potential for lutein production. J. Biotechnol. 2017, 262, 67–74. [Google Scholar] [CrossRef]
- Ferreira, V.S.; Pinto, R.F.; Sant’Anna, C. Low light intensity and nitrogen starvation modulate the chlorophyll content of Scenedesmus dimorphus. J. Appl. Microbiol. 2015, 120, 661–670. [Google Scholar] [CrossRef]
- Chen, D.M.; Li, J.; Dai, X.; Sun, Y.; Chen, F. Effect of phosphorus and temperature on chlorophyll a contents and cell sizes of Scenedesmus obliquus and Microcystis aeruginosa. Limnology 2011, 12, 187–192. [Google Scholar] [CrossRef]
- Masojídek, J.; Torzillo, G.; Koblízek, M.; Kopecký, J.; Bernardini, P.; Sacchi, A.; Komenda, J. Photoadaptation of two members of the Chlorophyta (Scenedesmus and Chlorella) in laboratory and outdoor cultures: Changes in chlorophyll fluorescence quenching and the xanthophyll cycle. Planta 1999, 209, 126–135. [Google Scholar] [CrossRef]
- Kuai, B.; Chen, J.; Hörtensteiner, S. The biochemistry and molecular biology of chlorophyll breakdown. J. Exp. Bot. 2018, 69, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, Y.; Shen, Y.; Yan, D.; He, X.; Dai, J.; Wu, Q. Oil accumulation mechanisms of the oleaginous microalga Chlorella protothecoides revealed through its genome, transcriptomes, and proteomes. BMC Genom. 2014, 10, 582. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Shimoda, Y.; Tanaka, A.; Ito, H. Chlorophyll a is a favorable substrate for Chlamydomonas Mg-dechelatase encoded by STAY-GREEN. Plant Physiol. Biochem. 2016, 109, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Guyer, L.; Schelbert Hofstetter, S.; Christ, B.; Silvestre Lira, B.; Rossi, M.; Hörtensteiner, S. Different Mechanisms Are Responsible for Chlorophyll Dephytylation during Fruit Ripening and Leaf Senescence in Tomato. Plant Physiol. 2014, 166, 44–56. [Google Scholar] [CrossRef]
- Engel, N.; Curty, C.; Gossauer, A. Chlorophyll catabolism in Chorella protothecoides. Part 8: Facts and artefacts. Plant Physiol. Biochem. 1996, 34, 77–83. [Google Scholar]
- Bale, N.J.; Llewellyn, C.A.; Airs, R.L. Atmospheric pressure chemical ionisation liquid chromatography/mass spectrometry of type II chlorophyll—A transformation products: Diagnostic fragmentation patterns. Org. Geochem. 2010, 41, 473–481. [Google Scholar] [CrossRef]
- Grabski, K.; Baranowski, N.; Skórko-Glonek, J.; Tukaj, Z. Chlorophyll catabolites in conditioned media of green microalga Desmodesmus subspicatus. J. Appl. Phycol. 2016, 28, 889–896. [Google Scholar] [CrossRef]
- Hynninen, P.H.; Hyvärinen, K. Tracing the Allomerization Pathways of Chlorophylls by 18O-Labeling and Mass Spectrometry. J. Org. Chem. 2002, 6712, 4055–4061. [Google Scholar] [CrossRef]
- Vergara-Domínguez, H.; Gandul-Rojas, B.; Roca, M. Formation of oxidised chlorophyll catabolites in olives. J. Food Compos. Anal. 2011, 24, 851–857. [Google Scholar] [CrossRef]
- Hynninen, P.H. Chemistry of chlorophylls: Modifications. In Chlorophylls; Scheer, H., Tsuchiya, T., Ohta, H., Okawa, K., Iwamatsu, A., Shimada, H., Masuda, T., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 145–209. [Google Scholar]
- Louda, W.; Mongkhonsri, P.; Baker, E.W. Chlorophyll degradation during senescence and death-III: 3–10 yr experiments, implications for ETIO series generation. Org. Geochem. 2011, 42, 688–699. [Google Scholar] [CrossRef]
- Walker, J.S.; Squier, A.H.; Hodgson, D.A.; Keely, B.J. Origin and significance of 132-hydroxychlorophyll derivatives in sediments. Org. Chem. 2002, 33, 1667–1674. [Google Scholar]
- Bale, N.J.; Airs, R.L.; Martin, P.; Lampitt, R.S.; Llewellyn, C.A. Chlorophyll-a transformations associated with sinking diatoms during termination of a North Atlantic spring bloom. Mar. Chem. 2015, 172, 23–33. [Google Scholar] [CrossRef]
- Steele, D.J.; Kimmance, S.A.; Franklin, D.J.; Airs, R.L. Occurrence of chlorophyll allomers during virus-induced mortality and population decline in the ubiquitous picoeukaryote Ostreococcus tauri. Environ. Microbiol. 2018, 20, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Steele, D.J.; Tarran, G.A.; Widdicombe, C.E.; Woodward, E.M.S.; Kimmance, S.A.; Franklin, D.J.; Airs, R.L. Abundance of a chlorophyll a precursor and the oxidation product hydroxychlorophyll a during seasonal phytoplankton community progression in the Western English Channel. Prog. Oceanogr. 2015, 137, 434–445. [Google Scholar] [CrossRef]
- Walker, J.S.; Keely, B.J. Distribution and significance of chlorophyll derivatives and oxidation products during the spring phytoplankton bloom in the Celtic Sea April 2002. Org. Geochem. 2004, 35, 1289–1298. [Google Scholar] [CrossRef]
- Naylor, C.C.; Keely, B.J. Sedimentary purpurins: Oxidative transformation products of chlorophylls. Org. Geochem. 1998, 28, 417–422. [Google Scholar] [CrossRef]
- Mulders, K.J.M.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef]
- Rippka, R.; Stanier, R.Y.; Deruelles, J.; Herdman, M.; Waterbury, J.B. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Maroneze, M.M.; Siqueira, S.F.; Vendruscolo, R.G.; Wagner, R.; de Menezes, C.R.; Zepka, L.Q.; Jacob-Lopes, E. The role of photoperiods on photobioreactors—A potential strategy to reduce costs. Bioresour. Technol. 2016, 219, 493–499. [Google Scholar] [CrossRef]
- Francisco, É.C.; Franco, T.T.; Wagner, R.; Jacob-Lopes, E. Assessment of different carbohydrates as exogenous carbon source in cultivation of cyanobacteria. Bioprocess Biosyst. Eng. 2014, 37, 1497–1505. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Wright, S.W.; Zapata, M. Microalgal Classes and their Signature Pigments. In Phytoplankton pigments; Roy, S., Llewellyn, C.A., Egeland, E.S., Johnsen, G., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 3–77. [Google Scholar]
- Chen, K.; Ríos, J.J.; Pérez, A.; Roca, M. Development of an accurate and high-throughput methodology for structural comprehension of chlorophylls derivatives. (I) Phytylated derivatives. J. Chromatogr. A 2015, 1406, 99–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breithaupt, D.E.; Wirt, U.; Bamedi, A. Differentiation between lutein monoester regioisomers and detection of lutein diesters from marigold flowers (Tagetes erecta, L.) and several fruits by liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2002, 50, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.J.; Xavier, A.A.O.; Díaz-Salido, E.; Arenilla-Vélez, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Pérez-Gálvez, A. Xanthophyll esters are found in human colostrum. Mol. Nutr. Food Res. 2017, 61, 1700296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paliwal, C.; Ghosh, T.; George, B.; Pancha, Y.; Maurya, R.; Chokshi, K.; Ghosh, A.; Mishra, S. Microalgal carotenoids: Potential nutraceutical compounds with chemotaxonomic importance. Algal Res. 2016, 15, 24–31. [Google Scholar] [CrossRef]
- Jahns, P.; Latowski, D.; Strzalka, K. Mechanism and regulation of the violaxanthin cycle: The role of antenna proteins and membrane lipids. Biochim. Biophys. Acta 2009, 1787, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Telfer, A.; Pascal, A.; Gall, A. Natural functions. In Carotenoids; Britton, G., Liaanen-Jensen, S., Pfander, H., Eds.; Birkhauser: Basel, Switzerland, 2008; Volume 4, pp. 189–211. [Google Scholar]
- Tanaka, R.; Tanaka, A. Chlorophyll cycle regulates the construction and destruction of the light-harvesting complexes. Biochim. Biophys. Acta 2011, 1807, 968–976. [Google Scholar] [CrossRef] [Green Version]
- Richardson, K.; Beardall, J.; Raven, J.A. Adaptation of Unicellular Algae to Irradiance: An Analysis of Strategies. New Phytol. 1983, 93, 157–191. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Owens, T.G. Light-Shade Adaptation. Two strategies in marine phytoplankton. Plant Physiol. 1980, 66, 592–595. [Google Scholar] [CrossRef] [Green Version]
- MacIntyre, H.L.; Kana, T.M.; Anning, T.; Geider, R.J. Photoacclimation of photosynthesis irradiance response curves and photosynthetic pigments in microalgae and cyanobacteria. J. Phycol. 2002, 38, 17–38. [Google Scholar] [CrossRef] [Green Version]
- Airs, R.L.; Keely, B.J. A high resolution study of the chlorophyll and bacteriochlorophyll pigment distributions in a calcite/gypsum microbial mat. Org. Geochem. 2003, 34, 539–551. [Google Scholar] [CrossRef]
- Cirulis, J.T.; Ashley Scott, J.; Ross, G.M. Management of oxidative stress by microalgae. Can. J. Physiol. Pharmacol. 2013, 91, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Hanumantha Rao, P.; Subramanian, V.V.; Sivasubramanian, V. Enzymatic and non-enzymatic antioxidant potentials of Chlorella vulgaris grown in effluent of a confectionery industry. J. Food Sci. Technol. 2014, 51, 322–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beisel, K.G.; Jahnke, S.; Hofmann, D.; Köppchen, S.; Schurr, U.; Matsubara, S. Continuous Turnover of Carotenes and Chlorophyll a in Mature Leaves of Arabidopsis Revealed by 14CO2 Pulse-Chase Labeling. Plant Physiol. 2010, 152, 2188–2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadnichuk, I.N.; Rakhimberdieva, M.G.; Bolychevtseva, Y.V.; Yurina, N.P.; Karapetyan, N.V.; Selyakh, I.O. Inhibition by glucose of chlorophyll a and phycocyanobilin biosynthesis in the unicellular red alga Galdieria partita at the stage of coproporphyrinogen III formation. Plant Sci. 1998, 136, 11–23. [Google Scholar] [CrossRef]
- Kamalanathan, M.; Dao, T.L.; Panjhaphol, C.; Gleadow, R.; Beardall, J. Photosynthetic physiology of Scenedesmus sp. under photoautotrophic, and molasses-based heterotrophic and mixotrophic conditions. Phycologia 2017, 56, 666–674. [Google Scholar] [CrossRef]
- Guyer, L.; Salinger, K.; Krügel, U.; Hörtensteiner, S. Catalytic and structural properties of pheophytinase, the phytol esterase involved in chlorophyll breakdown. J. Exp. Bot. 2018, 69, 879–889. [Google Scholar] [CrossRef] [Green Version]




| Parameter | Phototrophic | Heterotrophic |
|---|---|---|
| Xmax (mg/L) | 2650 ± 111.8 | 2600 ± 97.5 |
| µmax (h−1) | 0.023 ± 0.00 | 0.024 ± 0.00 |
| RT (h) | 216 ± 0.00 | 120 ± 0.00 |
| GT (h) | 30.13 ± 0.60 | 28.8 ± 0.49 |
| PX (mg/L h) | 10.87 ± 0.43 | 19.75 ± 0.29 |
| Time (h) | Neox | Violax | Luteox | Antherax | Lutein | β-Carotene | Total |
|---|---|---|---|---|---|---|---|
| 0 | + | + | + | 0.0 | 703.3 | 30.0 | 703.3 |
| 24 | 127.0 | 11.0 | 20.0 | 0.0 | 689.5 | 30.1 | 877.2 |
| 48 | 142.0 | 10.4 | 26.3 | 0.0 | 860.7 | 38.9 | 1174.3 |
| 72 | 130.3 | 28.1 | 37.0 | 7.0 | 795.4 | 45.9 | 1044.0 |
| 96 | 83.7 | 25.8 | 40.7 | 15.6 | 931.2 | 53.2 | 1150.1 |
| 120 | 146.7 | 28.5 | 36.7 | 20.4 | 1238.3 | 176.4 | 1647.0 |
| 144 | 147.0 | 40.0 | 45.1 | 32.0 | 1443.9 | 224.7 | 1952.4 |
| 168 | 122.3 | 19.8 | 51.3 | 26.2 | 1125.0 | 220.0 | 1679.6 |
| 192 | 156.2 | 38.9 | 67.0 | 30.0 | 1313.2 | 227.1 | 1832.4 |
| 216 | 321.9 | 108.9 | 44.0 | 49.2 | 1408.7 | 212.6 | 2145.3 |
| Time (h) | Pheo a | OH-Pheo a | OH-Chl a | Chl a | Phy a |
|---|---|---|---|---|---|
| 0 | 0.0 | 0.0 | 0.0 | 2438.3 | 2787.7 |
| 24 | 0.0 | 0.0 | 85.0 | 2420.0 | 3350.0 |
| 48 | 20.4 | 0.0 | 111.1 | 3955.2 | 2950.7 |
| 72 | 33.0 | 0.0 | 148.6 | 3718.6 | 2375.9 |
| 96 | 155.6 | 0.0 | 114.0 | 4079.3 | 2413.7 |
| 120 | 210.0 | 22.9 | 133.3 | 3981.3 | 2590.0 |
| 144 | 262.7 | 13.7 | 127.5 | 7417.5 | 897.8 |
| 168 | 86.7 | 8.3 | 134.6 | 5935.8 | 878.5 |
| 192 | 31.7 | 11.3 | 0.0 | 6170.0 | 708.7 |
| 216 | 8.5 | 0.0 | 0.0 | 6878.1 | 192.1 |
| Time (h) | OH-Lact.-Chl b | OH-Chl b | Chl b | Series a/b |
|---|---|---|---|---|
| 0 | 100.0 | 116.7 | 1408.3 | 3.22 |
| 24 | 175.0 | 124.5 | 1472.5 | 3.30 |
| 48 | 179.3 | 418.1 | 2023.0 | 2.71 |
| 72 | 275.1 | 437.6 | 5557.0 | 1.02 |
| 96 | 122.6 | 308.6 | 1757.2 | 3.02 |
| 120 | 116.7 | 310.4 | 1724.0 | 3.13 |
| 144 | 132.0 | 231.8 | 2016.7 | 3.55 |
| 168 | 90.0 | 286.3 | 1802.7 | 3.19 |
| 192 | 56.8 | 31.1 | 1878.3 | 3.50 |
| 216 | 40.9 | 50.6 | 1747.2 | 3.85 |
| 240 | 51.0 | 63.5 | 2038.6 | 3.14 |
| Pigment | Residence Time (h) | |||||
|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | 96 | 120 | |
| Neoxanthin | 86.4 | 76.9 | 80.0 | 43.5 | 36.5 | 13.3 |
| Violaxanthin | 42.2 | 18.7 | 18.4 | 10.4 | 8.5 | 1.8 |
| Lutein | 429.3 | 323.2 | 318.3 | 319.2 | 284.3 | 63.2 |
| β-carotene | 241.5 | 144.3 | 151.2 | 131.8 | 128.8 | 6.6 |
| Chld a | 3.4 | 3.5 | 3.5 | 3.5 | 8.5 | 108.0 |
| Pheo a | 78.3 | 157.7 | 145.0 | 67.2 | 37.2 | 299.9 |
| Chl b | 2284.0 | 2644.4 | 2778.2 | 1992.9 | 1164.0 | 895.4 |
| OH-chl a | 15.1 | 111.6 | 187.5 | 3.5 | 3.5 | 202.5 |
| Chl a | 4407.1 | 4343.0 | 4190.2 | 4636.3 | 2841.9 | 825.4 |
| Phy a | 1063.5 | 1102.6 | 766.1 | 326.4 | 262.0 | 207.0 |
| Tot. carot | 799.4 | 563.2 | 568.0 | 505.1 | 458.2 | 85.0 |
| Tot. chls | 7851.6 | 8362.9 | 8070.7 | 7030.1 | 4317.4 | 2538.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maroneze, M.M.; Zepka, L.Q.; Lopes, E.J.; Pérez-Gálvez, A.; Roca, M. Chlorophyll Oxidative Metabolism During the Phototrophic and Heterotrophic Growth of Scenedesmus obliquus. Antioxidants 2019, 8, 600. https://doi.org/10.3390/antiox8120600
Maroneze MM, Zepka LQ, Lopes EJ, Pérez-Gálvez A, Roca M. Chlorophyll Oxidative Metabolism During the Phototrophic and Heterotrophic Growth of Scenedesmus obliquus. Antioxidants. 2019; 8(12):600. https://doi.org/10.3390/antiox8120600
Chicago/Turabian StyleMaroneze, Mariana Manzoni, Leila Queiroz Zepka, Eduardo Jacob Lopes, Antonio Pérez-Gálvez, and María Roca. 2019. "Chlorophyll Oxidative Metabolism During the Phototrophic and Heterotrophic Growth of Scenedesmus obliquus" Antioxidants 8, no. 12: 600. https://doi.org/10.3390/antiox8120600
APA StyleMaroneze, M. M., Zepka, L. Q., Lopes, E. J., Pérez-Gálvez, A., & Roca, M. (2019). Chlorophyll Oxidative Metabolism During the Phototrophic and Heterotrophic Growth of Scenedesmus obliquus. Antioxidants, 8(12), 600. https://doi.org/10.3390/antiox8120600