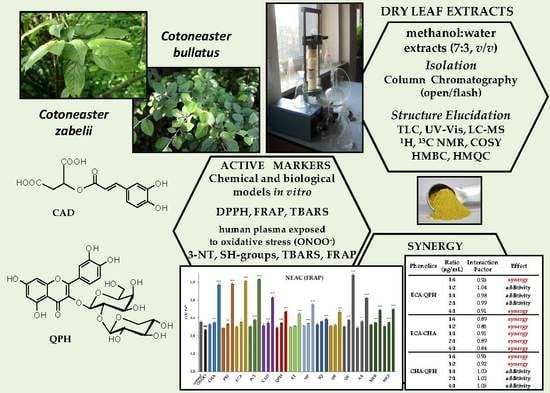
Contribution of Individual Polyphenols to Antioxidant Activity of Cotoneaster bullatus and Cotoneaster zabelii Leaves—Structural Relationships, Synergy Effects and Application for Quality Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. General
2.3. Extracts Preparation
2.4. Isolation of Polyphenols from Cotoneaster Leaf Extracts
2.5. Structure Elucidation
2.5.1. Acid Hydrolysis and Absolute Configuration of Monosaccharide Units in QPH
2.5.2. NMR Analysis
2.5.3. Quercetin 3--O-β-d-(2″--O-β-d-xylopyranosyl)galactopyranoside (QPH)
2.5.4. Caffeoylmalic Acid (CAD)
2.6. Quantitative HPLC-PDA Assay and Method Validation
2.7. Antioxidant Activity in Chemical Models
2.8. Antioxidant Activity in Human Plasma Model
2.9. Contribution of Individual Polyphenols to Antioxidant Activity of Cotoneaster Leaf Extracts
2.10. Interaction Effects of Combined Polyphenols
2.11. Statistical Analysis
3. Results and Discussion
3.1. Selection of Potential Active Markers
3.2. Isolation and Identification of Cotoneaster Polyphenols
3.3. Quantification of Phenolics
3.4. Antioxidant Activity of the Selected Markers in Chemical Models
3.5. Antioxidant Activity of the Selected Markers in Human Plasma Model
3.6. Contribution of Individual Polyphenols to Antioxidant Activity of the Extracts
3.7. Interactions of Model Polyphenols in the FRAP Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Holzer, V.M.; Lower-Nedza, A.D.; Nandintsetseg, M.; BatHokhuu, J.; Brantner, A.H. Antioxidant constituents of Cotoneaster melanocarpus Lodd. Antioxidants 2013, 2, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; López, V.; Caprioli, G.; Iannarelli, R.; Fiorini, D.; Innocenti, M.; Bellumori, M.; Maggi, F. Chemical constituents, radical scavenging activity and enzyme inhibitory capacity of fruits from Cotoneaster pannosus Franch. Food Funct. 2017, 8, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Uysal, A.; Gunes, E.; Aktumsek, A. Survey of phytochemical composition and biological effects of three extracts from a wild plant (Cotoneaster nummularia Fisch. et Mey.): A potential source for functional food ingredients and drug formulations. PLoS ONE 2014, 9, e113527. [Google Scholar] [CrossRef] [PubMed]
- Uysal, A.; Zengin, G.; Mollica, A.; Gunes, E.; Locatelli, M.; Yilmaz, T.; Aktumsek, A. Chemical and biological insights on Cotoneaster integerrimus: A new (–)-epicatechin source for food and medicinal applications. Phytomedicine 2016, 15, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Kicel, A.; Michel, P.; Owczarek, A.; Marchelak, A.; Żyżelewicz, D.; Budryn, G.; Oracz, J.; Olszewska, M.A. Phenolic profile and antioxidant potential of leaves from selected Cotoneaster Medik. species. Molecules 2016, 21, 688. [Google Scholar] [CrossRef] [Green Version]
- Kicel, A.; Kolodziejczyk-Czepas, J.; Owczarek, A.; Marchelak, A.; Sopinska, M.; Ciszewski, P.; Nowak, P.; Olszewska, M.A. Polyphenol-rich extracts from Cotoneaster leaves inhibit pro-inflammatory enzymes and protect human plasma components against oxidative stress in vitro. Molecules 2018, 23, 2472. [Google Scholar] [CrossRef] [Green Version]
- Kicel, A.; Kolodziejczyk-Czepas, J.; Owczarek, A.; Rutkowska, M.; Wajs-Bonikowska, A.; Granica, S.; Nowak, P.; Olszewska, M.A. Multifunctional phytocompounds in Cotoneaster fruits: Phytochemical profiling, cellular safety, anti-inflammatory and antioxidant effects in chemical and human plasma models in vitro. Oxid. Med. Cell. Longev. 2018, 2018, 1–16. [Google Scholar] [CrossRef]
- Sendker, J.; Sheridan, H. Composition and quality control of herbal medicines. In Toxicology Herbal Products; Pelkonen, O., Duez, P., Vuorela, P.M., Vuorela, H., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 29–65. [Google Scholar]
- Tistaert, C.; Dejaegher, B.; Vander Heyden, Y. Chromatographic separation techniques and data handling methods for herbal fingerprints: A review. Anal. Chim. Acta. 2011, 690, 148–161. [Google Scholar] [CrossRef]
- Pryor, W.A.; Cueto, R.; Jin, X.; Koppenol, W.H.; Ngu-Schwemlein, M.; Squadrito, G.L.; Uppu, P.L.; Uppu, R.M. A practical method for preparing peroxynitrite solutions of low ionic strength and free of hydrogen peroxide. Free Radic. Biol. Med. 1995, 18, 75–83. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Roj, J.M. Phenolic constituents of the inflorescences of Sorbus torminalis (L.) Crantz. Phytochem. Lett. 2011, 4, 151–157. [Google Scholar] [CrossRef]
- ICH, Q2(R1). Validation of Analytical Procedures: Text and Methodology; ICH: Geneva, Switzerland, 2005. [Google Scholar]
- Olszewska, M.A. New validated high-performance liquid chromatographic method for simultaneous analysis of ten flavonoid aglycones in plant extracts using a C18 fused-core column and acetonitrile-tetrahydrofuran gradient. J. Sep. Sci. 2012, 35, 2174–2183. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, M.A.; Michel, P. Antioxidant activity of inflorescences, leaves and fruits of three Sorbus species in relation to their polyphenolic composition. Nat. Prod. Res. 2009, 23, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk-Czepas, J.; Nowak, P.; Wachowicz, B.; Piechocka, J.; Głowacki, R.; Moniuszko-Szajwaj, B.; Stochmal, A. Antioxidant efficacy of Kalanchoe daigremontiana bufadienolide-rich fraction in blood plasma in vitro. Pharm. Biol. 2016, 54, 3182–3188. [Google Scholar] [CrossRef] [PubMed]
- Marchelak, A.; Owczarek, A.; Matczak, M.; Pawlak, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Bioactivity potential of Prunus spinosa L. flower extracts: Phytochemical profiling, cellular safety, pro-inflammatory enzymes inhibition and protective effects against oxidative stress in vitro. Front. Pharmacol. 2017, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Tallarida, R.J. Quantitative methods for assessing drug synergism. Genes Cancer 2011, 2, 1003–1008. [Google Scholar] [CrossRef] [Green Version]
- Biesaga, M. Influence of extraction methods on stability of flavonoids. J. Chromatogr. A 2011, 1218, 2505–2512. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Grayer, R.J.; Kite, G.C.; Veitich, N.C.; Eckert, M.R.; Marin, P.D.; Senanayake, S.P.; Paton, A.J. Leaf flavonoid glycosides as chemosystematic characters in Ocimum. Biochem. Syst. Ecol. 2002, 30, 327–342. [Google Scholar] [CrossRef]
- Kicel, A.; Wolbiś, M. Study on the phenolic constituents of the flowers and leaves of Trifolium repens L. Nat. Prod. Res. 2012, 26, 2050–2054. [Google Scholar] [CrossRef]
- Brandl, W.; Herrmann, K.; Grotjahn, L. Hydroxycinnamoyl esters of malic acid in small radish (Raphanus sativus L. var. sativus). Z. Naturforsch. 1984, 39, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.S.; Kim, H.K.; Lefeber, A.W.; Erkelens, C.; Choi, Y.H.; Verpoorte, R. Identification of phenylpropanoids in methyl jasmonate treated Brassica rapa leaves using two-dimensional nuclear magnetic resonance spectroscopy. J. Chromatogr. A 2006, 1112, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.S.; Li, Z.; Zheng, X.K.; Li, Y.J.; Su, F.Y.; Zhang, Y.L. Chemical constituents of Saxifraga stolonifera (L.) Meeb. Acta Pharm. Sin. 2010, 45, 742–746. [Google Scholar]
- Larsen, L.M.; Nielsen, J.K.; Sørensen, H. Identification of 3-O-[2-O-(β-D-xylopyranosyl)-β- D-galactopyranosyl] flavonoids in Horseradish leaves acting as feeding stimulants for a flea beetle. Phytochemistry 1982, 21, 1029–1033. [Google Scholar] [CrossRef]
- Hennebelle, T.; Sahpaz, S.; Ezer, N.; Bailleul, F. Polyphenols from Ballota larendana and Ballota pseudodictamnus. Biochem. Syst. Ecol. 2008, 36, 441–443. [Google Scholar] [CrossRef]
- Materska, M.; Olszowka, K.; Chilczuk, B.; Stochmal, A.; Pecio, Ł.; Pacholczyk-Sienicka, B.; Piacente, S.; Cosimo, P.; Masullo, M. Polyphenolic profiles in lettuce (Lactuca sativa L.) after CaCl2 treatment and cold storage. Eur. Food Res. Technol. 2019, 245, 733–744. [Google Scholar] [CrossRef] [Green Version]
- Olszewska, M. Flavonoids from Prunus serotina Ehrh. Acta Pol. Pharm. 2005, 62, 127–133. [Google Scholar]
- Soro, Y.; Kassi, A.B.B.; Bamba, F.; Siaka, S.; Toure, S.A.; Coustard, J.M. Flavonoids and gallic acid from leaves from Santaloides afzelii (Connaraceae). Rasayan J. Chem. 2012, 5, 332–337. [Google Scholar]
- Zhang, H.; Deng, A.; Zhang, Z.; Yu, Z.; Liu, Y.; Peng, S.; Wu, L.; Qin, H.; Wang, W. The protective effect of epicatechin on experimental ulcerative colitis in mice is mediated by increasing antioxidation and by the inhibition of NF-κB pathway. Pharmacol. Rep. 2016, 68, 514–520. [Google Scholar] [CrossRef]
- Shahat, A.A. Procyanidins from Adansonia digitata. Pharm. Biol. 2006, 44, 445–450. [Google Scholar] [CrossRef]
- De Bruyne, T.; Pieters, L.; Dommisse, R.; Kolodziej, H.; Wray, V.; Vanden Berghe, D.; Vlietinck, A. NMR characterization and biological evaluation of proanthocyanidins: A systematic approach. Basic Life Sci. 1999, 66, 193–209. [Google Scholar] [PubMed]
- Shoji, T.; Mutsuga, M.; Nakamura, T.; Kanda, T.; Akiyama, H.; Goda, Y. Isolation and structural elucidation of some procyanidins from apple by low-temperature nuclear magnetic resonance. J. Agric. Food Chem. 2003, 51, 3806–3813. [Google Scholar] [CrossRef] [PubMed]
- Taveniers, I.; De Loose, M.; Van Bockstaele, E. Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. Trends Anal. Chem. 2004, 23, 535–552. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muselík, J.; García-Alonso, M.; Martín-López, M.P.; Žemlička, M.; Rivas-Gonzalo, J.C. Measurement of antioxidant activity of wine catechins, procyanidins, anthocyanins and pyranoanthocyanins. Int. J. Mol. Sci. 2007, 8, 797–809. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Liu, R.H. Structure-activity relationships of flavonoids in the cellular antioxidant activity assay. J. Agric. Food Chem. 2008, 56, 8404–8411. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [Green Version]
- Graefe, E.U.; Wittig, J.; Mueller, S.; Riethling, A.K.; Uehleke, B.; Drewelow, B.; Pforte, H.; Jacobasch, G.; Derendorf, H.; Veit, M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 2001, 41, 492–499. [Google Scholar] [CrossRef]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [Green Version]
- Ou, K.; Gu, L. Absorption and metabolism of proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzynska, K. Investigation of antioxidant interaction between Green tea polyphenols and acetaminophen using isobolographic analysis. J. Pharm. Biomed. Anal. 2018, 159, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, M.; Olszewska, M.A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Owczarek, A. Sorbus domestica leaf extracts and their activity markers: Antioxidant potential and synergy effects in scavenging assays of multiple oxidants. Molecules 2019, 24, 2289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajimehdipoor, H.; Shahrestani, R.; Shekarchi, M. Investigating the synergistic antioxidant effects of some flavonoid and phenolic compounds. Res. J. Pharm. 2014, 1, 35–40. [Google Scholar]
- Pereira, R.B.; Sousa, C.; Costa, A.; Andrade, P.B.; Valentão, P. Glutathione and the antioxidant potential of binary mixtures with flavonoids: Synergisms and antagonisms. Molecules 2013, 18, 8858–8872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.H.; Wang, D.M.; Fan, S.F.; Li, D.W.; Luo, Z.W. Synergistic effects and related bioactive mechanism of Potentilla fruticosa L. leaves combined with Ginkgo biloba extracts studied with microbial test system (MTS). BMC Complement. Altern. Med. 2016, 16, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Analyte | tR | λ (nm) | Linear Range (µg/mL) | Regression | LOD (µg/mL) | LOQ (µg/mL) | Precision | Recovery (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Calibration Equation | n | r | Intra-Day (RSD, %) | Inter-Day (RSD, %) | |||||||
| NCHA | 3.58 | 325 | 1.85–110.5 | y = 14,045.0x | 6 | 0.9997 | 0.402 | 1.217 | 1.25 | 1.72 | 97.0 |
| CHA | 6.19 | 325 | 2.65–265.30 | y = 14,195.0x | 7 | 0.9997 | 0.397 | 1.204 | 1.40 | 1.75 | 100.5 |
| CCHA | 6.82 | 325 | 1.55–105.4 | y = 13,934.0x | 6 | 0.9998 | 0.405 | 1.227 | 1.30 | 1.65 | 98.4 |
| PB2 | 7.33 | 280 | 1.65–82.30 | y = 2399.4x | 6 | 0.9998 | 0.144 | 0.436 | 2.67 | 3.31 | 97.4 |
| ECA | 7.91 | 280 | 1.49–149.40 | y = 3367.1x | 7 | 0.9999 | 0.077 | 0.233 | 2.02 | 2.63 | 99.2 |
| PC1 | 8.71 | 280 | 1.52–78.30 | y = 2299.8x | 6 | 0.9997 | 0.135 | 0.403 | 2.70 | 3.51 | 98.6 |
| CAD | 9.12 | 328 | 1.92–95.80 | y = 5251.7x | 6 | 0.9997 | 0.071 | 0.220 | 1.35 | 1.69 | 97.5 |
| QPH | 9.54 | 350 | 2.16–08.20 | y = 6398.0x | 6 | 0.9998 | 0.018 | 0.054 | 1.83 | 2.01 | 98.6 |
| RT | 10.23 | 350 | 1.15–114.90 | y = 7135.3x | 7 | 0.9998 | 0.062 | 0.187 | 1.30 | 1.61 | 99.3 |
| HP | 10.49 | 350 | 2.51–251.20 | y = 9942.0x | 7 | 0.9998 | 0.129 | 0.391 | 1.25 | 1.63 | 98.2 |
| IQ | 10.85 | 350 | 1.39–69.60 | y = 9786.2x | 6 | 0.9997 | 0.047 | 0.142 | 1.78 | 1.94 | 96.9 |
| QR | 11.83 | 350 | 1.01–50.60 | y = 8929.3x | 6 | 0.9997 | 0.006 | 0.017 | 1.93 | 2.16 | 97.1 |
| Analyte | MEB | MEZ | Fraction | MEB | MEZ |
|---|---|---|---|---|---|
| mg/g dw | mg/g dw | ||||
| NCHA | 3.56 ± 0.11 C | 1.98 ± 0.02 A | TCA | 23.46 ± 1.57 | 47.36 ± 0.54 |
| CHA | 16.30 ± 0.92 H | 9.14 ± 0.13 F | TFL | 18.95 ± 0.94 | 14.91 ± 0.05 |
| CCHA | 3.46 ± 0.18 C | 2.67 ± 0.05 B | TLPA | 53.71 ± 3.78 | 39.76 ± 0.50 |
| PB2 | 17.60 ± 1.20 I | 11.93 ± 0.22 G | TPH | 96.12 ± 5.19 | 102.03 ± 1.09 |
| ECA | 12.07 ± 0.78 F | 8.89 ± 0.13 E | TPA (CYE) | 239.62 ± 12.36 | 241.84 ± 7.27 |
| PC1 | 14.28 ± 0.85 G | 8.88 ± 0.10 E | |||
| CAD | - | 31.83 ± 0.32 H | |||
| QPH | 7.50 ± 0.36 E | - | |||
| RT | 0.26 ± 0.01 A | 3.36 ± 0.02 C | |||
| HP | 5.66 ± 0.28 D | 1.72 ± 0.02 A | |||
| IQ | 0.80 ± 0.04 B | 2.36 ± 0.02 B | |||
| QR | 3.30 ± 0.17 C | 4.34 ± 0.04 D | |||
| Analyte | Radical Scavenging Activity DPPH a | Reducing Power b | LA-Peroxidation TBARS c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SC50 | TE | FRAP | TE | IC50 | TE | ||||
| µg/mL | µmol/L | mmol TE/g | mmol Fe2+/g | mol/mol | mmol TE/g | µg/mL | µmol/L | mmol TE/g | |
| CHA | 4.60 ± 0.07 G | 12.97 ± 0.21 F | 3.51 ± 0.05 B,C | 25.68 ± 0.49 E | 9.10 ± 0.18 E | 9.75 ± 0.26 G | 2.49 ± 0.01 A,B | 6.83 ± 0.08 D | 13.65 ± 0.19 G,H |
| PB2 | 2.46 ± 0.03 B | 4.25 ± 0.05 B | 6.57 ± 0.08 G | 32.64 ± 0.80 F | 18.89 ± 0.47 H | 12.63 ± 0.37 H | 2.56 ± 0.18 A,B | 4.43 ± 0.32 B | 13.28 ± 0.95 G,H |
| ECA | 2.35 ± 0.10 B | 8.08 ± 0.50 E | 6.89 ± 0.41 G,H | 35.79 ± 0.93 G | 10.39 ± 0.27 F | 13.97 ± 0.43 H | 2.25 ± 0.19 A | 7.73 ± 0.66 E | 15.16 ± 1.29 H |
| PC1 | 2.55 ± 0.11 B | 2.94 ± 0.09 A | 6.34 ± 0.28 G | 21.08 ± 0.80 C | 18.27 ± 0.06 H | 7.35 ± 0.37 D,E | 2.91 ± 0.08 A,B | 3.35 ± 0.09 A | 11.68 ± 0.31 F,G |
| CAD | 3.76 ± 0.11 E | 12.47 ± 0.37 F | 4.38 ± 0.13 D,E | 22.31 ± 1.22 C,D | 6.61 ± 0.36 C | 7.76 ± 0.56 E,F | 3.53 ± 0.28 B,C | 11.90 ± 0.93 G | 9.65 ± 0.76 E,F |
| QPH | 5.09 ± -0.08 H | 8.54 ± 0.14 E | 3.18 ± 0.05 B | 15.71 ± 0.20 B | 9.37 ± 0.12 E | 5.98 ± 0.13 C,D | 5.00 ± 0.24 D,E,F | 8.39 ± 0.40 E | 6.79 ± 0.33 C,D |
| RT | 4.44 ± 0.05 G | 7.27 ± 0.08 D | 3.64 ± 0.04 B,C | 15.68 ± 0.62 B | 9.57 ± 0.38 E | 5.79 ± 0.32 C | 6.01 ± 0.46 F | 9.84 ± 0.75 F | 5.66 ± 0.43 B,C |
| HP | 3.42 ± 0.07 C,D,E | 7.83 ± 0.16 D | 4.73 ± 0.10 E,F | 20.64 ± 0.02 C | 9.01 ± 0.01 E | 7.87 ± 0.08 E,F | 5.43 ± 0.62 E,F | 12.55 ± 1.28 G | 6.29 ± 0.72 C,D |
| IQ | 3.55 ± 0.08 D,E | 8.12 ± 0.21 E | 4.56 ± 0.10 D,E | 19.69 ± 0.27 C | 8.59 ± 0.06 D | 7.37 ± 0.11 D,E | 5.89 ± 0.09 F | 13.50 ± 0.36 H | 5.76 ± 0.09 B,C |
| QR | 3.31 ± 0.03 C,D | 7.39 ± 0.08 D | 4.88 ± 0.05 E,F | 20.26 ± 0.36 C | 9.08 ± 0.16 E | 7.78 ± 0.17 E,F | 4.15 ± 0.10 C,D | 9.04 ± 0.52 F | 8.18 ± 0.20 D,E |
| QU | 1.85 ± 0.11 A | 6.31 ± 0.33 C | 8.74 ± 0.07 H | 46.24 ± 1.66 H | 13.98 ± 0.50 G | 18.10 ± 0.99 I | 1.76 ± 0.06 A | 5.89 ± 0.29 C | 19.28 ± 0.62 I |
| TX | 4.06 ± 0.11F | 16.20 ± 0.42 G | 3.99 ± 0.10 C,D | 12.69 ± 0.42 B | 3.18 ± 0.11 A | 4.31 ± 0.19 B | 8.51 ± 0.74 G | 33.66 ± 2.48 J | 4.00 ± 0.35 A,B |
| AA | 3.13 ± 0.05 C | 17.63 ± 0.04 H | 5.17 ± 0.08 F | 25.26 ± 0.15 D,E | 4.45 ± 0.01 B | 9.19 ± 0.01 F,G | 4.59 ± 0.27 C,D,E | 26.06 ± 0.80 I | 7.40 ± 0.43 C,D,E |
| MEB | 7.19 ± 0.32 I | - | 3.06 ± 0.14 A | 10.74 ± 0.07 A | - | 3.76 ± 0.03 A | 10.58 ± 0.32 H | - | 3.21 ± 0.10 A |
| MEZ | 7.44 ± 0.03 I | - | 2.96 ± 0.01 A | 9.42 ± 013 A | - | 3.15 ± 0.06 A | 11.43 ± 0.71 H | - | 2.97 ± 0.18 A |
| Analyte | Ferric Reducing Antioxidant Power (FRAP) | |||||
|---|---|---|---|---|---|---|
| Chemical Model | Human Plasma Model | |||||
| AE a (g/g) | CTCB b (%) | CTCZ b (%) | AE a (g/g) | CTCB b (%) | CTCZ b (%) | |
| NCHA | 1.02 | 0.85 | 0.54 | 1.45 | 0.82 | 0.44 |
| CHA | 1.02 | 3.90 | 2.49 | 1.45 | 3.77 | 2.03 |
| CCHA | 1.02 | 0.83 | 0.73 | 1.45 | 0.80 | 0.59 |
| PB2 | 1.29 | 5.35 | 4.12 | 1.47 | 4.12 | 2.68 |
| ECA | 1.42 | 4.03 | 3.38 | 1.57 | 3.01 | 2.13 |
| PC1 | 0.83 | 2.81 | 1.99 | 1.63 | 3.69 | 2.21 |
| CAD | 0.88 | - | 7.53 | 1.04 | - | 5.04 |
| QPH | 0.62 | 1.10 | - | 0.60 | 0.71 | - |
| RT | 0.62 | 0.04 | 0.57 | 0.51 | 0.02 | 0.27 |
| HP | 0.82 | 1.10 | 0.37 | 0.80 | 0.73 | 0.21 |
| IQ | 0.78 | 0.17 | 0.50 | 0.32 | 0.04 | 0.12 |
| QR | 0.80 | 0.62 | 0.92 | 0.58 | 0.30 | 0.38 |
| TCA | - | 5.61 | 11.77 | - | 5.42 | 8.49 |
| TFL | - | 3.30 | 3.05 | - | 1.99 | 1.36 |
| TLPA | - | 14.10 | 11.75 | - | 13.34 | 9.53 |
| TPH | - | 23.01 | 26.57 | - | 20.75 | 19.38 |
| TPA | - | 47.03 | 54.12 | - | 61.89 | 60.17 |
| MEB | 0.38 | 100 | - | 0.63 | 100 | - |
| MEZ | 0.32 | - | 100 | 0.65 | - | 100 |
| Phenolic Mixture | Concentration Ratio (µg/mL) | Theoretical Effective Concentration (µg/mL) | Experimental Effective Concentration (µg/mL) | IF ± 95% Conf. | Effect |
|---|---|---|---|---|---|
| ECA:QPH | 1:4 | 2.09 | 1.81 ± 0.01 | 0.93 ± 0.01 | synergy |
| 1:2 | 1.80 | 1.81 ± 0.07 | 1.04 ± 0.04 | additivity | |
| 1:1 | 1.74 | 1.52 ± 0.01 | 0.98 ± 0.01 | additivity | |
| 2:1 | 1.48 | 1.38 ± 0.01 | 0.99 ± 0.01 | additivity | |
| 4:1 | 1.39 | 1.17 ± 0.01 | 0.91 ± 0.01 | synergy | |
| ECA:CHA | 1:4 | 1.56 | 1.36 ± 0.03 | 0.89 ± 0.02 | synergy |
| 1:2 | 1.39 | 1.23 ± 0.01 | 0.85 ± 0.01 | synergy | |
| 1:1 | 1.41 | 1.24 ± 0.01 | 0.91 ± 0.01 | synergy | |
| 2:1 | 1.25 | 1.15 ± 0.08 | 0.89 ± 0.06 | synergy | |
| 4:1 | 1.26 | 1.04 ± 0.13 | 0.84 ± 0.10 | synergy | |
| CHA:QPH | 1:4 | 2.19 | 2.00 ± 0.02 | 0.93 ± 0.01 | synergy |
| 1:2 | 1.97 | 1.89 ± 0.02 | 0.92 ± 0.01 | synergy | |
| 1:1 | 1.99 | 1.99 ± 0.09 | 1.03 ± 0.05 | additivity | |
| 2:1 | 1.78 | 1.87 ± 0.09 | 1.02 ± 0.05 | additivity | |
| 4:1 | 1.79 | 1.81 ± 0.19 | 1.03 ± 0.11 | additivity |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kicel, A.; Owczarek, A.; Kapusta, P.; Kolodziejczyk-Czepas, J.; Olszewska, M.A. Contribution of Individual Polyphenols to Antioxidant Activity of Cotoneaster bullatus and Cotoneaster zabelii Leaves—Structural Relationships, Synergy Effects and Application for Quality Control. Antioxidants 2020, 9, 69. https://doi.org/10.3390/antiox9010069
Kicel A, Owczarek A, Kapusta P, Kolodziejczyk-Czepas J, Olszewska MA. Contribution of Individual Polyphenols to Antioxidant Activity of Cotoneaster bullatus and Cotoneaster zabelii Leaves—Structural Relationships, Synergy Effects and Application for Quality Control. Antioxidants. 2020; 9(1):69. https://doi.org/10.3390/antiox9010069
Chicago/Turabian StyleKicel, Agnieszka, Aleksandra Owczarek, Paulina Kapusta, Joanna Kolodziejczyk-Czepas, and Monika A. Olszewska. 2020. "Contribution of Individual Polyphenols to Antioxidant Activity of Cotoneaster bullatus and Cotoneaster zabelii Leaves—Structural Relationships, Synergy Effects and Application for Quality Control" Antioxidants 9, no. 1: 69. https://doi.org/10.3390/antiox9010069
APA StyleKicel, A., Owczarek, A., Kapusta, P., Kolodziejczyk-Czepas, J., & Olszewska, M. A. (2020). Contribution of Individual Polyphenols to Antioxidant Activity of Cotoneaster bullatus and Cotoneaster zabelii Leaves—Structural Relationships, Synergy Effects and Application for Quality Control. Antioxidants, 9(1), 69. https://doi.org/10.3390/antiox9010069