Neurosporaxanthin Overproduction by Fusarium fujikuroi and Evaluation of Its Antioxidant Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Analyses of Carotenoids
2.3. Antioxidant Assays
2.3.1. DPPH and FRAP Assays
2.3.2. Quenching Activity
2.3.3. Scavenging Activity in Liposomes
3. Results
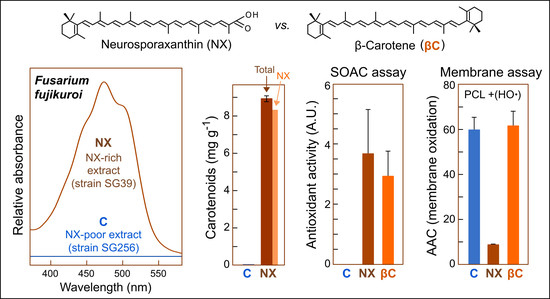
3.1. Development of Conditions for Improved NX Production
3.2. Obtaining of NX-Enriched Extracts for Antioxidant Assays
3.3. Antioxidant Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Concepcion, M.R.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Carmen Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avalos, J.; Nordzieke, S.; Parra, O.; Pardo-Medina, J.; Limón, M.C. Carotenoid production by filamentous fungi and yeasts. In Biotechnology of Yeasts and Filamentous Fungi; Sibirny, A.A., Ed.; Springer: Cham, Switzerland, 2017; pp. 225–279. ISBN 978-3-319-58828-5. [Google Scholar]
- Avalos, J.; Cerdá-Olmedo, E. Fungal carotenoid production. In Handbook of Fungal Biotechnology; Arora, D.K., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2004; pp. 367–378. [Google Scholar]
- Aasen, A.J.; Jensen, S.L. Fungal carotenoids Π. The structure of the carotenoid acid neurosporaxanthin. Acta Chem. Scand 1965, 19, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Avalos, J.; Corrochano, L.M. Carotenoid biosynthesis in Neurospora. In Neurospora: Genomics and Molecular Biology; Kasbekar, D.P., McCluskey, K., Eds.; Caister Academic Press: Norfolk, UK, 2013; pp. 227–241. [Google Scholar]
- Avalos, J.; Díaz-Sánchez, V.; García-Martínez, J.; Castrillo, M.; Ruger-Herreros, M.; Limón, M.C. Carotenoids. In Biosynthesis and Molecular Genetics of Fungal Secondary Metabolites; Martín, J.F., García-Estrada, C., Zeilinger, S., Eds.; Springer: New York, NY, USA, 2014; pp. 149–185. [Google Scholar]
- Bindl, E.; Lang, W.; Rau, W. Untersuchungen über die lichtabhängige Carotinoidsynthese. VI. Zeitlicher Verlauf der Synthese der einzelnen Carotinoide bei Fusarium aquaeductuum unter verschiedenen Induktionsbedingungen. Planta 1970, 94, 156–174. [Google Scholar] [CrossRef]
- Avalos, J.; Cerdá-Olmedo, E. Chemical modification of carotenogenesis in Gibberella fujikuroi. Phytochemistry 1986, 25, 1837–1841. [Google Scholar] [CrossRef]
- Rodríguez-Ortiz, R.; Michielse, C.; Rep, M.; Limón, M.C.; Avalos, J. Genetic basis of carotenoid overproduction in Fusarium oxysporum. Fungal Genet. Biol. 2012, 49, 684–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valadon, L.R.G.; Mummery, R.S. Biosynthesis of neurosporaxanthin. Microbios 1969, 1, 3–8. [Google Scholar]
- Valadon, L.R.G.; Osman, M.; Mummery, R.S.; Jerebzoff-Quintin, S.; Jerebzoff, S. The effect of monochromatic radiation in the range 350 to 750 nm on the carotenogenesis in Verticillium agaricinum. Physiol. Plant. 1982, 56, 199–203. [Google Scholar] [CrossRef]
- Strobel, I.; Breitenbach, J.; Scheckhuber, C.Q.; Osiewacz, H.D.; Sandmann, G. Carotenoids and carotenogenic genes in Podospora anserina: Engineering of the carotenoid composition extends the life span of the mycelium. Curr. Genet. 2009, 55, 175–184. [Google Scholar] [CrossRef]
- Cen, Y.-K.; Lin, J.-G.; Wang, Y.-L.; Wang, J.-Y.; Liu, Z.-Q.; Zheng, Y.-G. The gibberellin producer Fusarium fujikuroi: Methods and technologies in the current toolkit. Front. Bioeng. Biotechnol. 2020, 8, 232. [Google Scholar] [CrossRef]
- Avalos, J.; Pardo-Medina, J.; Parra-Rivero, O.; Ruger-Herreros, M.; Rodríguez-Ortiz, R.; Hornero-Méndez, D.; Limón, M.C. Carotenoid biosynthesis in Fusarium. J. Fungi 2017, 3, 39. [Google Scholar] [CrossRef] [Green Version]
- Prado-Cabrero, A.; Schaub, P.; Díaz-Sánchez, V.; Estrada, A.F.; Al-Babili, S.; Avalos, J. Deviation of the neurosporaxanthin pathway towards β-carotene biosynthesis in Fusarium fujikuroi by a point mutation in the phytoene desaturase gene. FEBS J. 2009, 276, 4582–4597. [Google Scholar] [CrossRef] [PubMed]
- Prado-Cabrero, A.; Estrada, A.F.; Al-Babili, S.; Avalos, J. Identification and biochemical characterization of a novel carotenoid oxygenase: Elucidation of the cleavage step in the Fusarium carotenoid pathway. Mol. Microbiol. 2007, 64, 448–460. [Google Scholar] [CrossRef]
- Díaz-Sánchez, V.; Estrada, A.F.; Trautmann, D.; Al-Babili, S.; Avalos, J. The gene carD encodes the aldehyde dehydrogenase responsible for neurosporaxanthin biosynthesis in Fusarium fujikuroi. FEBS J. 2011, 278, 3164–3176. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.M.; Prado-Cabrero, A.; Fernández-Martín, R.; Avalos, J. A gene of the opsin family in the carotenoid gene cluster of Fusarium fujikuroi. Curr. Genet. 2004, 46, 47–58. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, J.; Brunk, M.; Avalos, J.; Terpitz, U. The CarO rhodopsin of the fungus Fusarium fujikuroi is a light-driven proton pump that retards spore germination. Sci. Rep. 2015, 5, 7798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adam, A.; Deimel, S.; Pardo-Medina, J.; García-Martínez, J.; Konte, T.; Limón, M.C.; Avalos, J.; Terpitz, U. Protein activity of the Fusarium fujikuroi rhodopsins CarO and OpsA and their relation to fungus-plant interaction. Int. J. Mol. Sci. 2018, 19, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada, A.F.; Avalos, J. Regulation and targeted mutation of opsA, coding for the NOP-1 opsin orthologue in Fusarium fujikuroi. J. Mol. Biol. 2009, 387, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Avalos, J.; Estrada, A.F. Regulation by light in Fusarium. Fungal Genet. Biol. 2010, 47, 930–938. [Google Scholar] [CrossRef]
- Avalos, J.; Cerdá-Olmedo, E. Carotenoid mutants of Gibberella fujikuroi. Curr. Genet. 1987, 11, 505–511. [Google Scholar] [CrossRef]
- Rodríguez-Ortiz, R.; Limón, M.C.; Avalos, J. Regulation of carotenogenesis and secondary metabolism by nitrogen in wild-type Fusarium fujikuroi and carotenoid-overproducing mutants. Appl. Environ. Microbiol. 2009, 75, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Ortiz, R.; Limón, M.C.; Avalos, J. Functional analysis of the carS gene of Fusarium fujikuroi. Mol. Genet. Genom. 2013, 288, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Ruger-Herreros, M.; Parra-Rivero, O.; Pardo-Medina, J.; Romero-Campero, F.J.; Limón, M.C.; Avalos, J. Comparative transcriptomic analysis unveils interactions between the regulatory CarS protein and light response in Fusarium. BMC Genom. 2019, 20, 67. [Google Scholar] [CrossRef] [PubMed]
- Avalos, J.; Limón, M.C. Biological roles of fungal carotenoids. Curr. Genet. 2014, 61, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Iigusa, H.; Yoshida, Y.; Hasunuma, K. Oxygen and hydrogen peroxide enhance light-induced carotenoid synthesis in Neurospora crassa. FEBS Lett. 2005, 579, 4012–4016. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, Y.; Hasunuma, K. Reactive oxygen species affect photomorphogenesis in Neurospora crassa. J. Biol. Chem. 2004, 279, 6986–6993. [Google Scholar] [CrossRef] [Green Version]
- Di Mascio, P.; Sundquist, A.R.; Devasagayam, T.P.A.; Sies, H. Assay of lycopene and other carotenoids as singlet oxygen quenchers. In Methods in Enzymology; Carotenoids Part A: Chemistry, Separation, Quantitation, and Antioxidation; Academic Press: New York, NY, USA, 1992; Volume 213, pp. 429–438. [Google Scholar]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Aizawa, K.; Iwasaki, Y.; Ouchi, A.; Inakuma, T.; Nagaoka, S.; Terao, J.; Mukai, K. Development of singlet oxygen absorption capacity (SOAC) assay method. 2. Measurements of the SOAC values for carotenoids and food extracts. J. Agric. Food Chem. 2011, 59, 3717–3729. [Google Scholar] [CrossRef]
- Ouchi, A.; Aizawa, K.; Iwasaki, Y.; Inakuma, T.; Terao, J.; Nagaoka, S.-I.; Mukai, K. Kinetic study of the quenching reaction of singlet oxygen by carotenoids and food extracts in solution. Development of a singlet oxygen absorption capacity (SOAC) assay method. J. Agric. Food Chem. 2010, 58, 9967–9978. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Takahashi, S.; Aizawa, K.; Mukai, K. Development of singlet oxygen absorption capacity (SOAC) assay method. 4. Measurements of the SOAC values for vegetable and fruit extracts. Biosci. Biotechnol. Biochem. 2015, 79, 280–291. [Google Scholar] [CrossRef]
- Mukai, K.; Ouchi, A.; Azuma, N.; Takahashi, S.; Aizawa, K.; Nagaoka, S.-I. Development of a singlet oxygen absorption capacity (SOAC) assay method. Measurements of the SOAC values for carotenoids and α-tocopherol in an aqueous triton X-100 micellar solution. J. Agric. Food Chem. 2017, 65, 784–792. [Google Scholar] [CrossRef]
- Wisniewska, A.; Widomska, J.; Subczynski, W.K. Carotenoid-membrane interactions in liposomes: Effect of dipolar, monopolar, and nonpolar carotenoids. Acta Biochim. Pol. 2006, 53, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Geissman, T.; Verbiscar, A.; Phinney, B.; Cragg, G. Studies on the biosynthesis of gibberellins from (−)-kaurenoic acid in cultures of Gibberella fujikuroi. Phytochemistry 1966, 5, 933–947. [Google Scholar] [CrossRef]
- Avalos, J.; Prado-Cabrero, A.; Estrada, A.F. Neurosporaxanthin production by Neurospora and Fusarium. Methods Mol. Biol. 2012, 898, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Estrada, A.F.; Avalos, J. The White Collar protein WcoA of Fusarium fujikuroi is not essential for photocarotenogenesis, but is involved in the regulation of secondary metabolism and conidiation. Fungal Genet. Biol. 2008, 45, 705–718. [Google Scholar] [CrossRef]
- Hornero-Méndez, D.; Limón, M.C.; Avalos, J. HPLC analysis of carotenoids in neurosporaxanthin-producing fungi. In Microbial Carotenoids: Methods and Protocols; Methods in Molecular Biology; Barreiro, C., Barredo, J.-L., Eds.; Springer: New York, NY, USA, 2018; pp. 269–281. [Google Scholar]
- Drummen, G.P.C.; van Liebergen, L.C.M.; Op den Kamp, J.A.F.; Post, J.A. C11-BODIPY581/591, an oxidation-sensitive fluorescent lipid peroxidation probe: (micro) spectroscopic characterization and validation of methodology. Free. Radic. Biol. Med. 2002, 33, 473–490. [Google Scholar] [CrossRef]
- Rodríguez-Ortiz, R.; Mehta, B.J.; Avalos, J.; Limón, M.C. Stimulation of bikaverin production by sucrose and by salt starvation in Fusarium fujikuroi. Appl. Microbiol. Biotechnol. 2010, 85, 1991–2000. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Luque, E.M.; Gutiérrez, G.; Navarro-Sampedro, L.; Olmedo, M.; Rodríguez-Romero, J.; Ruger-Herreros, C.; Tagua, V.G.; Corrochano, L.M. A relationship between carotenoid accumulation and the distribution of species of the fungus Neurospora in Spain. PLoS ONE 2012, 7, e33658. [Google Scholar] [CrossRef] [Green Version]
- Kudryavtseva, O.A.; Mazheika, I.S.; Solovchenko, A.E.; Kamzolkina, O.V. Genetic instability of the short-living ascomycetous fungus Podospora anserina induced by prolonged submerged cultivation. Microbiology 2011, 80, 784–796. [Google Scholar] [CrossRef]
- Zalokar, M. Studies on biosynthesis of carotenoids in Neurospora crassa. Arch. Biochem. Biophys. 1954, 50, 71–80. [Google Scholar] [CrossRef]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Adholeya, A. A review on the assessment of stress conditions for simultaneous production of microalgal lipids and carotenoids. Front. Microbiol. 2016, 7, 546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youssar, L.; Schmidhauser, T.J.; Avalos, J. The Neurospora crassa gene responsible for the cut and ovc phenotypes encodes a protein of the haloacid dehalogenase family. Mol. Microbiol. 2005, 55, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Sokolovsky, V.Y.; Lauter, F.-R.; Müller-Röber, B.; Ricci, M.; Schmidhauser, T.J.; Russo, V.E.A. Nitrogen regulation of blue light-inducible genes in Neurospora crassa. J. Gen. Microbiol. 1992, 138, 2045–2049. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.Y.; Keasling, J.D. Amplification of HMG-CoA reductase production enhances carotenoid accumulation in Neurospora crassa. Metab. Eng. 2002, 4, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, N.H.; Davalos, A.F.; Puente, P.A.; Rodríguez, A.Y.; Caicedo, P.A. Antioxidant activity of exo-metabolites produced by Fusarium oxysporum: An endophytic fungus isolated from leaves of Otoba gracilipes. MicrobiologyOpen 2019, 8, e903. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Tian, Y.-X.; Yang, F.; Zhang, J.-P.; Skibsted, L.H. Antioxidant synergism between carotenoids in membranes. Astaxanthin as a radical transfer bridge. Food Chem. 2009, 115, 1437–1442. [Google Scholar] [CrossRef]
- Mukai, K.; Daifuku, K.; Okabe, K.; Tanigaki, T.; Inoue, K. Structure-activity relationship in the quenching reaction of singlet oxygen by tocopherol (vitamin E) derivatives and related phenols. Finding of linear correlation between the rates of quenching of singlet oxygen and scavenging of peroxyl and phenoxyl radicals in solution. J. Org. Chem. 1991, 56, 4188–4192. [Google Scholar] [CrossRef]
- Nagai, S.; Ohara, K.; Mukai, K. Kinetic study of the quenching reaction of singlet oxygen by flavonoids in ethanol solution. J. Phys. Chem. B 2005, 109, 4234–4240. [Google Scholar] [CrossRef]
- Takahashi, S.; Tsutsumi, A.; Aizawa, K.; Suganuma, H. Kinetic study of the quenching reaction of singlet oxygen by flavonoids in ethanol solution. Food Sci. Technol. Res. 2018, 24, 921–933. [Google Scholar] [CrossRef]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Mortensen, A.; Skibsted, L.H.; Truscott, T.G. The interaction of dietary carotenoids with radical species. Biochem. Biophys. 2001, 385, 13–19. [Google Scholar] [CrossRef]
- Tay-Agbozo, S.; Street, S.; Kispert, L.D. The carotenoid bixin: Optical studies of aggregation in polar/water solvents. J. Photochem. Photobiol. A Chem. 2018, 362, 31–39. [Google Scholar] [CrossRef]
- Fuciman, M.; Durchan, M.; Šlouf, V.; Keşan, G.; Polívka, T. Excited-state dynamics of astaxanthin aggregates. Chem. Phys. Lett. 2013, 568, 21–25. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Bala, T.; Prasad, B.L.V.; Sastry, M.; Kahaly, M.U.; Waghmare, U.V. Interaction of different metal ions with carboxylic acid group: A quantitative study. J. Phys. Chem. A 2007, 111, 6183–6190. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, N.E.; Focsan, A.L.; Bowman, M.K.; Kispert, L.D. Free radical formation in novel carotenoid metal ion complexes of astaxanthin. J. Phys. Chem. B 2010, 114, 16968–16977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Focsan, A.L.; Polyakov, N.E.; Kispert, L.D. Supramolecular carotenoid complexes of enhanced solubility and stability-The way of bioavailability improvement. Molecules 2019, 24, 3947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mano, C.M.; Guaratini, T.; Cardozo, K.H.M.; Colepicolo, P.; Bechara, E.J.; Barros, M.P. Astaxanthin restrains nitrative-oxidative peroxidation in mitochondrial-mimetic liposomes: A pre-apoptosis model. Mar. Drugs 2018, 16, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grudzinski, W.; Nierzwicki, L.; Welc, R.; Reszczynska, E.; Luchowski, R.; Czub, J.; Gruszecki, W.I. Localization and orientation of xanthophylls in a lipid bilayer. Sci. Rep. 2017, 7, 9619. [Google Scholar] [CrossRef] [PubMed]
- Lancrajan, I.; Diehl, H.A.; Socaciu, C.; Engelke, M.; Zorn-Kruppa, M. Carotenoid incorporation into natural membranes from artificial carriers: Liposomes and β-cyclodextrins. Chem. Phys. Lipids 2001, 112, 1–10. [Google Scholar] [CrossRef]
- Barros, M.P.; Rodrigo, M.J.; Zacarias, L. Dietary carotenoid roles in redox homeostasis and human health. J. Agric. Food Chem. 2018, 66, 5733–5740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasnokutskaya, I.S.; Leontieva, S.V.; Finkelshtein, E.I.; Khodonov, A.A.; Shvets, V.I. Effect of carbonyls on the reactivity of polyenes in autoxidation. J. Phys. Org. Chem. 2003, 16, 226–231. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra-Rivero, O.; Paes de Barros, M.; Prado, M.d.M.; Gil, J.-V.; Hornero-Méndez, D.; Zacarías, L.; Rodrigo, M.J.; Limón, M.C.; Avalos, J. Neurosporaxanthin Overproduction by Fusarium fujikuroi and Evaluation of Its Antioxidant Properties. Antioxidants 2020, 9, 528. https://doi.org/10.3390/antiox9060528
Parra-Rivero O, Paes de Barros M, Prado MdM, Gil J-V, Hornero-Méndez D, Zacarías L, Rodrigo MJ, Limón MC, Avalos J. Neurosporaxanthin Overproduction by Fusarium fujikuroi and Evaluation of Its Antioxidant Properties. Antioxidants. 2020; 9(6):528. https://doi.org/10.3390/antiox9060528
Chicago/Turabian StyleParra-Rivero, Obdulia, Marcelo Paes de Barros, María del Mar Prado, José-Vicente Gil, Dámaso Hornero-Méndez, Lorenzo Zacarías, María J. Rodrigo, M. Carmen Limón, and Javier Avalos. 2020. "Neurosporaxanthin Overproduction by Fusarium fujikuroi and Evaluation of Its Antioxidant Properties" Antioxidants 9, no. 6: 528. https://doi.org/10.3390/antiox9060528
APA StyleParra-Rivero, O., Paes de Barros, M., Prado, M. d. M., Gil, J.-V., Hornero-Méndez, D., Zacarías, L., Rodrigo, M. J., Limón, M. C., & Avalos, J. (2020). Neurosporaxanthin Overproduction by Fusarium fujikuroi and Evaluation of Its Antioxidant Properties. Antioxidants, 9(6), 528. https://doi.org/10.3390/antiox9060528