Doubly Phosphorylated Peptide Vaccines to Protect Transgenic P301S Mice against Alzheimer’s Disease Like Tau Aggregation
Abstract
:1. Introduction
2. Experimental
2.1. Solid Phase Peptide Synthesis
2.2. Synthesis of Phospho-Tau Peptide Vaccines
| T cell epitope | Origin | Sequence * |
|---|---|---|
| TT | Tetanus toxin 582–599 of Clostridium tetani | VDDALINSTKIYSYFPSV |
| TBC | Ag85B 241–255 of Mycobacterium tuberculosis | QDAYNAGGGHNAVFD |
| B cell epitope | ||
| Tau199–208[pS202/pT205] | hMAPT | SPGpSPGpTPGS |
| Tau209–217[pT212/pS214] | hMAPT | RSRpTPpSLPT |
| Tau229–237[pT231/pS235] | hMAPT | VRpTPPKpSPS |
2.3. Peptide Cleavage, Purification and Analysis
2.4. Animals
2.5. Immunization

2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Behavioral Characterization
2.7.1. Wire Hang Test
2.7.2. Accelerating Rotarod Test
2.7.3. Beam Walk Test
2.8. Primary Monoclonal Anti-phospho Tau Antibodies (mAb)
2.9. Immunohistochemistry
2.10. Preparation of Brain Homogenates
2.11. Relative Quantification of Phospho-Tau
2.12. Immunoblot Analysis
2.13. Statistical Data Analysis
3. Results
3.1. Design and Peptide Synthesis of Immunogens

| B cell epitope | T cell epitope | tR [min] | MMcalc/MMobs | Yield [%] | Purity [%] |
|---|---|---|---|---|---|
| Tau199–208[pS202/pT205] | TT | 31.5 | 3368.6/3368.7 | 15.4 | 90 |
| TBC | 15.3 | 2858.2/2858.1 | 13.8 | 90 | |
| Tau209–217[pT212/pS214] | TT | 26.5 | 3539.8/3539.7 | 21.8 | 95 |
| TBC | 18.8 | 3029.4/3029.4 | 22.8 | 85 | |
| Tau229–237[pT231/pS235] | TT | 21.8 | 3493.8/3493.8 | 10.4 | >95 |
| TBC | 26.9 | 2983.4/2983.6 | 9.8 | 95 |
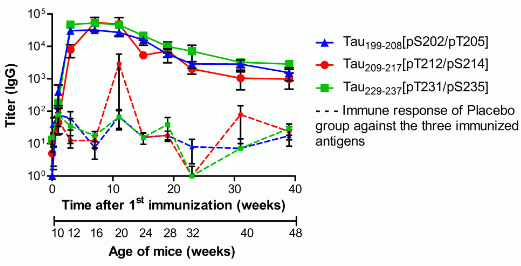
3.2. Specific Total IgG Titers
3.3. Specific IgG1/IgG2a/IgG2b/IgG2c Titers
 , n = 39), Tau209-217[pT212/pS214] (red circle
, n = 39), Tau209-217[pT212/pS214] (red circle  , n = 39) or Tau229–237[pT231/pS235] (green square
, n = 39) or Tau229–237[pT231/pS235] (green square  , n = 36). Animals were immunized and boosted twice, two and six weeks after. Sera were collected one week before (background) and again 1, 3, 7, 11, 15, 19, 23, 31, and 39 weeks after the first vaccination (day 0). Solid lines represent the IgG titers of vaccinated mice; dashed lines represent the unspecific immune response against the three antigens, which was detected in sera of placebo treated mice (n = 36). Shown are geometric means of IgG titers with the standard error of the mean (SEM).
, n = 36). Animals were immunized and boosted twice, two and six weeks after. Sera were collected one week before (background) and again 1, 3, 7, 11, 15, 19, 23, 31, and 39 weeks after the first vaccination (day 0). Solid lines represent the IgG titers of vaccinated mice; dashed lines represent the unspecific immune response against the three antigens, which was detected in sera of placebo treated mice (n = 36). Shown are geometric means of IgG titers with the standard error of the mean (SEM).
 , n = 39), Tau209-217[pT212/pS214] (red circle
, n = 39), Tau209-217[pT212/pS214] (red circle  , n = 39) or Tau229–237[pT231/pS235] (green square
, n = 39) or Tau229–237[pT231/pS235] (green square  , n = 36). Animals were immunized and boosted twice, two and six weeks after. Sera were collected one week before (background) and again 1, 3, 7, 11, 15, 19, 23, 31, and 39 weeks after the first vaccination (day 0). Solid lines represent the IgG titers of vaccinated mice; dashed lines represent the unspecific immune response against the three antigens, which was detected in sera of placebo treated mice (n = 36). Shown are geometric means of IgG titers with the standard error of the mean (SEM).
, n = 36). Animals were immunized and boosted twice, two and six weeks after. Sera were collected one week before (background) and again 1, 3, 7, 11, 15, 19, 23, 31, and 39 weeks after the first vaccination (day 0). Solid lines represent the IgG titers of vaccinated mice; dashed lines represent the unspecific immune response against the three antigens, which was detected in sera of placebo treated mice (n = 36). Shown are geometric means of IgG titers with the standard error of the mean (SEM).
3.4. Tau Phosphorylation Degrees in Brain Homogenates

 ), Tau209–217[pT212/pS214] (n = 5–6,
), Tau209–217[pT212/pS214] (n = 5–6,  ), and Tau229–237[pT231/pS235] (n = 5–6,
), and Tau229–237[pT231/pS235] (n = 5–6,  ) at 32 weeks (upper row) and 48 weeks (lower row) of age obtained from immunoblots. Shown are the relative ratios of phospho-tau (mAbs AT8, AT100, and AT180) to total tau (mAb Tau5) in percent arranged according to the temporal occurrence of the phospho-tau epitopes (pT231/pS235—early, pT212/pS214—intermediate, pS202/pT205—late).
) at 32 weeks (upper row) and 48 weeks (lower row) of age obtained from immunoblots. Shown are the relative ratios of phospho-tau (mAbs AT8, AT100, and AT180) to total tau (mAb Tau5) in percent arranged according to the temporal occurrence of the phospho-tau epitopes (pT231/pS235—early, pT212/pS214—intermediate, pS202/pT205—late).
 ), Tau209–217[pT212/pS214] (n = 5–6,
), Tau209–217[pT212/pS214] (n = 5–6,  ), and Tau229–237[pT231/pS235] (n = 5–6,
), and Tau229–237[pT231/pS235] (n = 5–6,  ) at 32 weeks (upper row) and 48 weeks (lower row) of age obtained from immunoblots. Shown are the relative ratios of phospho-tau (mAbs AT8, AT100, and AT180) to total tau (mAb Tau5) in percent arranged according to the temporal occurrence of the phospho-tau epitopes (pT231/pS235—early, pT212/pS214—intermediate, pS202/pT205—late).
) at 32 weeks (upper row) and 48 weeks (lower row) of age obtained from immunoblots. Shown are the relative ratios of phospho-tau (mAbs AT8, AT100, and AT180) to total tau (mAb Tau5) in percent arranged according to the temporal occurrence of the phospho-tau epitopes (pT231/pS235—early, pT212/pS214—intermediate, pS202/pT205—late).
3.5. Immunohistochemical Quantification of Tau Pathology in P301S Mice
 ), Tau209–217[pT212/pS214] (n = 9,
), Tau209–217[pT212/pS214] (n = 9,  ), or Tau229–237[pT231/pS235] (n = 9,
), or Tau229–237[pT231/pS235] (n = 9,  ).
).
 ), Tau209–217[pT212/pS214] (n = 9,
), Tau209–217[pT212/pS214] (n = 9,  ), or Tau229–237[pT231/pS235] (n = 9,
), or Tau229–237[pT231/pS235] (n = 9,  ).
).
3.6. Behavioral Characterization of P301S Mice
 , n = 15), Tau209–217[pT212/pS214] (
, n = 15), Tau209–217[pT212/pS214] (  , n = 16), or Tau229–237[pT231/pS235] (
, n = 16), or Tau229–237[pT231/pS235] (  , n = 20). (A) body weight; (B) photograph of a paralyzed P301S mouse; (C) survival rates; (D) percentage of paralysis. Statistical significances are marked by asterisks (***, p < 0.001).
, n = 20). (A) body weight; (B) photograph of a paralyzed P301S mouse; (C) survival rates; (D) percentage of paralysis. Statistical significances are marked by asterisks (***, p < 0.001).
 , n = 15), Tau209–217[pT212/pS214] (
, n = 15), Tau209–217[pT212/pS214] (  , n = 16), or Tau229–237[pT231/pS235] (
, n = 16), or Tau229–237[pT231/pS235] (  , n = 20). (A) body weight; (B) photograph of a paralyzed P301S mouse; (C) survival rates; (D) percentage of paralysis. Statistical significances are marked by asterisks (***, p < 0.001).
, n = 20). (A) body weight; (B) photograph of a paralyzed P301S mouse; (C) survival rates; (D) percentage of paralysis. Statistical significances are marked by asterisks (***, p < 0.001).

4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miniño, A.M.; Xu, J.; Kochanek, K.D. Deaths: Preliminary Data for 2008. In National Vital Statistics Reports; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2010; Volume 59. [Google Scholar]
- Selkoe, D.J. Amyloid beta protein precursor and the pathogenesis of Alzheimer’s disease. Cell 1989, 58, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Physiological production of the beta-amyloid protein and the mechanism of Alzheimer’s disease. Trends Neurosci. 1993, 16, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Grundke-Iqbal, I.; Iqbal, K.; Tung, Y.C.; Quinlan, M.; Wisniewski, H.M.; Binder, L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA 1986, 83, 4913–4917. [Google Scholar] [CrossRef] [PubMed]
- Kosik, K.S.; Joachim, C.L.; Selkoe, D.J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1986, 83, 4044–4048. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.G.; Mirra, S.S.; Pollock, N.J.; Binder, L.I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau). Proc. Natl. Acad. Sci. USA 1986, 83, 4040–4043. [Google Scholar] [CrossRef] [PubMed]
- Mandelkow, E.-M.; Mandelkow, E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb. Perspect. Med. 2012, 2. [Google Scholar] [CrossRef]
- Wang, W.; Fan, L.; Xu, D.; Wen, Z.; Yu, R.; Ma, Q. Immunotherapy for Alzheimer’s disease. Acta Biochim. Biophys. Sin. 2012, 44, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, Y.; Lee, V.M.Y.; Trojanowski, J.Q. Therapeutic strategies for tau mediated neurodegeneration. J. Neurol. Neurosurg. Psychiatry 2013, 84, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Bulic, B.; Pickhardt, M.; Mandelkow, E.-M.; Mandelkow, E. Tau protein and tau aggregation inhibitors. Neuropharmacology 2010, 59, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Brunden, K.R.; Ballatore, C.; Crowe, A.; Smith, A.B.; Lee, V.M.-Y.; Trojanowski, J.Q. Tau-directed drug discovery for Alzheimer’s disease and related tauopathies: A focus on tau assembly inhibitors. Exp. Neurol. 2010, 223, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Delrieu, J.; Ousset, P.J.; Caillaud, C.; Vellas, B. “Clinical trials in Alzheimer’s disease”: Immunotherapy approaches. J. Neurochem. 2012, 120, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Asuni, A.A.; Boutajangout, A.; Quartermain, D.; Sigurdsson, E.M. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J. Neurosci. 2007, 27, 9115–9129. [Google Scholar] [CrossRef] [PubMed]
- Boimel, M.; Grigoriadis, N.; Lourbopoulos, A.; Haber, E.; Abramsky, O.; Rosenmann, H. Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice. Exp. Neurol. 2010, 224, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Boutajangout, A.; Ingadottir, J.; Davies, P.; Sigurdsson, E.M. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J. Neurochem. 2011, 118, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Boutajangout, A.; Quartermain, D.; Sigurdsson, E.M. Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J. Neurosci. 2010, 30, 16559–16566. [Google Scholar] [CrossRef] [PubMed]
- Troquier, L.; Caillierez, R.; Burnouf, S.; Fernandez-Gomez, F.J.; Grosjean, M.-E.; Zommer, N.; Sergeant, N.; Schraen-Maschke, S.; Blum, D.; Buee, L. Targeting phospho-Ser422 by active Tau immunotherapy in the THYTau22 mouse model: A suitable therapeutic approach. Curr. Alzheimer Res. 2012, 9, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.I.; Mozdzanowska, K.; Williams, K.L.; Singer, D.; Richter, M.; Hoffmann, R.; Caton, A.J.; Otvos, L.; Erikson, J.; Tompkins, S.M. Vaccination with M2e-Based Multiple antigenic peptides: characterization of the B cell response and protection efficacy in inbred and outbred mice. PLoS One 2011, 6, e28445. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, R.; Dakappagari, N.K.; Kaumaya, P.T.P. Synthetic peptides as cancer vaccines. Biopolymers 2002, 66, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Menendez-Gonzalez, M.; Perez-Pinera, P.; Martinez-Rivera, M.; Muniz, A.L.; Vega, J.A. Immunotherapy for Alzheimer’s disease: Rational basis in ongoing clinical trials. Curr. Pharm. Des. 2011, 17, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Federoff, H.J. Therapeutic potential of vaccines for Alzheimer’s disease. Immunotherapy 2011, 3, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Erb, P.; Troxler, M.; Fluri, M.; Grogg, D.; Alkan, S.S. Functional heterogeneity of CD4-positive T-cell subsets: The correlation between effector functions and lymphokine secretion is limited. Cell. Immunol. 1991, 135, 232–244. [Google Scholar] [PubMed]
- Infante, A.J.; Currier, P.F. Collaboration of Th1 and Th2 T cell clones in specific antibody responses: Regulation of the IgM response to phosphorylcholine. Cell. Immunol. 1989, 122, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, E.M.; Knudsen, E.; Asuni, A.; Fitzer-Attas, C.; Sage, D.; Quartermain, D.; Goni, F.; Frangione, B.; Wisniewski, T. An attenuated immune response is sufficient to enhance cognition in an Alzheimer’s disease mouse model immunized with amyloid-beta derivatives. J. Neurosci. 2004, 24, 6277–6282. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, V.; Tanga, F.Y.; DeLeo, J.A. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur. J. Neurosci. 2004, 20, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Rozenstein-Tsalkovich, L.; Grigoriadis, N.; Lourbopoulos, A.; Nousiopoulou, E.; Kassis, I.; Abramsky, O.; Karussis, D.; Rosenmann, H. Repeated immunization of mice with phosphorylated-tau peptides causes neuroinflammation. Exp. Neurol. 2013, 248, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Bungener, L.; Geeraedts, F.; ter Veer, W.; Medema, J.; Wilschut, J.; Huckriede, A. Alum boosts TH2-type antibody responses to whole-inactivated virus influenza vaccine in mice but does not confer superior protection. Vaccine 2008, 26, 2350–2359. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Hoffmann, R.; Singer, D. T-cell epitope-dependent immune response in inbred (C57BL/6J, SJL/J, and C3H/HeN) and transgenic P301S and Tg2576 mice. J. Pept. Sci. 2013, 19, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Maurin, H.; Chong, S.-A.; Kraev, I.; Davies, H.; Kremer, A.; Seymour, C.M.; Lechat, B.; Jaworski, T.; Borghgraef, P.; Devijver, H.; et al. Early structural and functional defects in synapses and myelinated axons in stratum lacunosum moleculare in two preclinical models for tauopathy. PLoS One 2014, 9, e87605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levenga, J.; Krishnamurthy, P.; Rajamohamedsait, H.; Wong, H.; Franke, T.F.; Cain, P.; Sigurdsson, E.M.; Hoeffer, C.A. Tau pathology induces loss of GABAergic interneurons leading to altered synaptic plasticity and behavioral impairments. Acta Neuropathol. Commun. 2013, 1. [Google Scholar] [CrossRef]
- Singer, D.; Volke, D.; Hoffmann, R. Characterization of phosphorylation dependent antibodies to study the phosphorylation status of the Tau protein. Int. J. Pept. Res. Ther. 2005, 11, 279–289. [Google Scholar] [CrossRef]
- Yoshiyama, Y.; Higuchi, M.; Zhang, B.; Huang, S.-M.; Iwata, N.; Saido, T.C.; Maeda, J.; Suhara, T.; Trojanowski, J.Q.; Lee, V.M.-Y. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 2007, 53, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, E.M.; Scholtzova, H.; Mehta, P.D.; Frangione, B.; Wisniewski, T. Immunization with a nontoxic/nonfibrillar amyloid-beta homologous peptide reduces Alzheimer’s disease-associated pathology in transgenic mice. Am. J. Pathol. 2001, 159, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Singer, D.; Lehmann, J.; Hanisch, K.; Hartig, W.; Hoffmann, R. Neighbored phosphorylation sites as PHF-tau specific markers in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2006, 346, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Iba, M.; Inoue, H.; Higuchi, M.; Takao, K.; Tsukita, K.; Karatsu, Y.; Iwamoto, Y.; Miyakawa, T.; Suhara, T. P301S mutant human tau transgenic mice manifest early symptoms of human tauopathies with dementia and altered sensorimotor gating. PLoS One 2011, 6, e21050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, R.J.; Morton, J.; Dunnett, S.B. Motor coordination and balance in rodents. Curr. Protoc. Neurosci. 2001, 8. [Google Scholar] [CrossRef]
- Bailey, K.R.; Crawley, J.N. Chapter 5 Anxiety-Related Behaviors in Mice. In Methods of Behavior Analysis in Neuroscience, 2nd ed.; Buccafusco, J.J., Ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Porzig, R.; Singer, D.; Hoffmann, R. Epitope mapping of mAbs AT8 and Tau5 directed against hyperphosphorylated regions of the human tau protein. Biochem. Biophys. Res. Commun. 2007, 358, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.; Lee, V.M.-Y.; Leight, S.; Varga, I.; Otvos, L., Jr. Unique Alzheimer’s disease paired helical filament specific epitopes involve double phosphorylation at specific sites. Biochemistry 1997, 36, 8114–8124. [Google Scholar] [CrossRef] [PubMed]
- Hiesberger, T.; Trommsdorff, M.; Howell, B.W.; Goffinet, A.; Mumby, M.C.; Cooper, J.A.; Herz, J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation. Neuron 1999, 24, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Hanger, D.P.; Anderton, B.H.; Noble, W. Tau phosphorylation: The therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 2009, 15, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- ImageJ. version 1.46r. U.S. National Institutes of Health: Bethesda, MD, USA, 2012.
- Rabilloud, T. Use of thiourea to increase the solubility of membrane proteins in two-dimensional electrophoresis. Electrophoresis 1998, 19, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.M.; Riederer, B.M. Sample preparation for two-dimensional gel electrophoresis. Proteomics 2003, 3, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- GraphPad Prism Software. version 5.02 for Windows. GraphPad Software Inc.: San Diego, CA, USA, 2009.
- Kaumaya, P.T.P.; Berndt, K.D.; Heidorn, D.B.; Trewhella, J.; Kezdy, F.J.; Goldberg, E. Synthesis and biophysical characterization of engineered topographic immunogenic determinants with alpha alpha topology. Biochemistry 1990, 29, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Dumont, M.; Stack, C.; Elipenahli, C.; Jainuddin, S.; Gerges, M.; Starkova, N.N.; Yang, L.; Starkov, A.A.; Beal, F. Behavioral deficit, oxidative stress, and mitochondrial dysfunction precede tau pathology in P301S transgenic mice. FASEB J. 2011, 25, 4063–4072. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Cirrito, J.R.; Stewart, F.R.; Jiang, H.; Finn, M.B.; Holmes, B.B.; Binder, L.I.; Mandelkow, E.-M.; Diamond, M.I.; Lee, V.M.-Y. In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J. Neurosci. 2011, 31, 13110–13117. [Google Scholar] [CrossRef] [PubMed]
- Yanamandra, K.; Kfoury, N.; Jiang, H.; Mahan, T.E.; Ma, S.; Maloney, S.E.; Wozniak, D.F.; Diamond, M.I.; Holtzman, D.M. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron 2013, 80, 402–414. [Google Scholar] [CrossRef]
- Study of Intravenous Immunoglobulin in Amnestic Mild Cognitive Impairment. Available online: http://clinicaltrials.gov/ (accessed on 2 February 2014).
- Study Evaluating The Efficacy And Safety Of Bapineuzumab In Alzheimer Disease Patients. Available online: http://clinicaltrials.gov/ (accessed on 2 February 2014).
- Study Evaluating the Safety and Efficacy of Bapineuzumab in Alzheimer Disease Patients. Available online: http://clinicaltrials.gov/ (accessed on 2 February 2014).
- Study Evaluating The Safety Of AAB-003 (PF-05236812). In Subjects With Alzheimerߣs Disease. Available online: http://clinicaltrials.gov/ (accessed on 2 February 2014).
- Open Label Extension Study Evaluating Safety and Tolerability of AAB-003 (PF-05236812) in Subject With Mild to Moderate Alzheimerߣs Disease. Available online: http://clinicaltrials.gov/ (accessed on 2 February 2014).
- Bi, M.; Ittner, A.; Ke, Y.D.; Götz, J.; Ittner, L.M.; Ferreira, S.T. Tau-targeted immunization impedes progression of neurofibrillary histopathology in aged P301L tau transgenic mice. PLoS One 2011, 6, e26860. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Wu, S.; Murray, T.K.; Kinley, R.; Cella, C.V.; Sims, H.; Buckner, N.; Hanmer, J.; Davies, P.; O’Neill, M.J.; et al. Passive immunization with anti-tau antibodies in two transgenic models: Reduction of tau pathology and delay of disease progression. J. Biol. Chem. 2011, 286, 34457–34467. [Google Scholar] [CrossRef] [PubMed]
- Rosenmann, H.; Grigoriadis, N.; Karussis, D.; Boimel, M.; Touloumi, O.; Ovadia, H.; Abramsky, O. Tauopathy-like Abnormalities and neurologic deficits in mice immunized with neuronal tau protein. Arch Neurol. 2006, 63, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Agger, E.M.; Cassidy, J.P.; Brady, J.; Korsholm, K.S.; Vingsbo-Lundberg, C.; Andersen, P. Adjuvant modulation of the cytokine balance in Mycobacterium tuberculosis subunit vaccines; immunity, pathology and protection. Immunology 2008, 124, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Marrack, P.; McKee, A.S.; Munks, M.W. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 2009, 9, 287–293. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Richter, M.; Mewes, A.; Fritsch, M.; Krügel, U.; Hoffmann, R.; Singer, D. Doubly Phosphorylated Peptide Vaccines to Protect Transgenic P301S Mice against Alzheimer’s Disease Like Tau Aggregation. Vaccines 2014, 2, 601-623. https://doi.org/10.3390/vaccines2030601
Richter M, Mewes A, Fritsch M, Krügel U, Hoffmann R, Singer D. Doubly Phosphorylated Peptide Vaccines to Protect Transgenic P301S Mice against Alzheimer’s Disease Like Tau Aggregation. Vaccines. 2014; 2(3):601-623. https://doi.org/10.3390/vaccines2030601
Chicago/Turabian StyleRichter, Monique, Agneta Mewes, Manuela Fritsch, Ute Krügel, Ralf Hoffmann, and David Singer. 2014. "Doubly Phosphorylated Peptide Vaccines to Protect Transgenic P301S Mice against Alzheimer’s Disease Like Tau Aggregation" Vaccines 2, no. 3: 601-623. https://doi.org/10.3390/vaccines2030601
APA StyleRichter, M., Mewes, A., Fritsch, M., Krügel, U., Hoffmann, R., & Singer, D. (2014). Doubly Phosphorylated Peptide Vaccines to Protect Transgenic P301S Mice against Alzheimer’s Disease Like Tau Aggregation. Vaccines, 2(3), 601-623. https://doi.org/10.3390/vaccines2030601