Measurement of Multi Ion Transport through Human Bronchial Epithelial Cell Line Provides an Insight into the Mechanism of Defective Water Transport in Cystic Fibrosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Cells
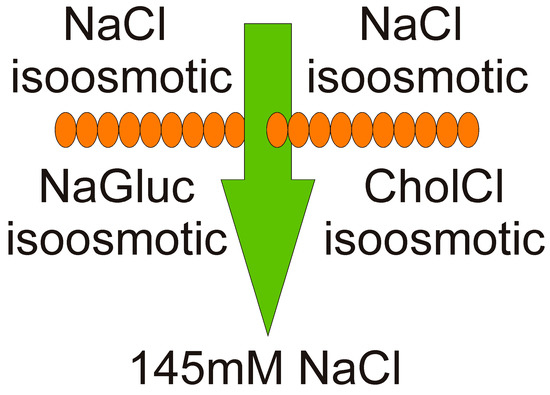
2.3. The Media
2.4. Electrodes
2.5. Measurement of Multiple Ion Transport Procedure
3. Results
4. Discussion
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hollenhorst, M.I.; Richter, K.; Fronius, M. Ion Transport by Pulmonary Epithelia. J. Biomed. Biotechnol. 2011, 2011, 174306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toczylowska-Maminska, R.; Dolowy, K. Ion transporting proteins of human bronchial epithelium. J. Cell. Biochem. 2012, 113, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Reichhart, N.; Strauss, O. Ion channels and transporters of the retinal pigment epithelium. Exp. Eye Res. 2014, 126, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Mall, M.A.; Galietta, L.J.V. Targeting ion channels in cystic fibrosis. J. Cyst. Fibros. 2015, 14, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Bartoszewski, R.; Matalon, S.; Collawn, J.F. Ion channels of the lung and their role in disease pathogenesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L859–L872. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Praetorius, J. Fundamentals of Bicarbonate Secretion in Epithelia. In Ion Channels and Transporters of Epithelia in Health and Disease. Physiology in Health and Disease; Hamilton, K., Devor, D., Eds.; Springer: New York, NY, USA, 2016; pp. 187–263. [Google Scholar] [CrossRef]
- Webster, M.J.; Tarran, R. Slippery When Wet: Airway Surface Liquid Homeostasis and Mucus Hydration. Cell Vol. Regul. 2018, 81, 293–335. [Google Scholar] [CrossRef]
- Zajac, M.; Dolowy, K. Measurement of ion fluxes across epithelia. Prog. Biophys. Mol. Biol. 2017, 127, 1–11. [Google Scholar] [CrossRef]
- Munkonge, F.; Alton, E.W.; Andersson, C.; Davidson, H.; Dragomir, A.; Edelman, A.; Farley, R.; Hjelte, L.; McLachlan, G.; Stern, M.; et al. Measurement of halide efflux from cultured and primary airway epithelial cells using fluorescence indicators. J. Cyst. Fibros. 2004, 3, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Toczylowska-Maminska, R.; Lewenstam, A.; Dolowy, K. Multielectrode Bisensor System for Time-Resolved Monitoring of Ion Transport Across an Epithelial Cell Layer. Anal. Chem. 2014, 86, 390–394. [Google Scholar] [CrossRef]
- Zajac, M.; Lewenstam, A.; Dolowy, K. Multi-electrode system for measurement of transmembrane ion-fluxes through living epithelial cells. Bioelectrochemistry 2017, 117, 65–73. [Google Scholar] [CrossRef]
- Zajac, M.; Lewenstam, A.; Stobiecka, M.; Dolowy, K. New ISE-based apparatus for Na+, K+, Cl−, pH and transepithelial potential difference real-time simultaneous measurements of ion transport across epithelial cells monolayer-advantages and pitfalls. Sensors 2019, 19, 1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anagnostopoulou, P.; Dai, L.; Schatterny, J.; Hirtz, S.; Duerr, J.; Mall, M.A. Allergic airway inflammation induces a pro-secretory epithelial ion transport phenotype in mice. Eur. Respir. J. 2010, 36, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Schultz, B.D.; DeRoos, A.D.G.; Venglarik, C.J.; Singh, A.K.; Frizzell, R.A.; Bridges, R.J. Glibenclamide blockade of CFTR chloride channels. Am. J. Physiol. Lung Cell. Mol. Physiol. 1996, 271, L192–L200. [Google Scholar] [CrossRef]
- Okada, Y.; Sato, K.; Numata, T. Pathophysiology and puzzles of the volume-sensitive outwardly rectifying anion channel. J. Physiol. 2009, 587, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.; Lee, H.; Lee, N.; Choi, Y.C. Evidence that basolateral but not apical membrane localization of outwardly rectifying depolarization induced Cl channel in airway epithelia. J. Membr. Biol. 2000, 176, 217–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szkotak, A.J.; Man, S.F.P.; Duszyk, M. The role of basolateral outwardly rectifying chloride channel in human airway epithelial anion secretion. Am. J. Respir. Cell Mol. Biol. 2003, 29, 710–720. [Google Scholar] [CrossRef]
- Blaisdell, C.J.; Edmonds, R.D.; Wang, X.T.; Guggino, S.; Zeitlin, P.L. pH-regulated chloride secretion in fetal lung epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, L1248–L1255. [Google Scholar] [CrossRef]
- Tresguerres, M.; Buck, J.; Levin, L.R. Physiological carbon dioxide, bicarbonate, and pH sensing. Pflügers Arch.-Eur. J. Physiol. 2010, 460, 953–964. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Kumaran, G.K.; Hanukoglu, I. High-resolution imaging af the actin cytoskeleton and epithelial sodium channel, CFTR, and aquaporin-9 localization in the vas deferens. Mol. Reprod. Dev. 2020, 1–15. [Google Scholar] [CrossRef]
- Boucher, R.C. Human airway ion transport. Part one. Am. J. Respir. Crit. Care Med. 1994, 150, 271–281. [Google Scholar] [CrossRef]
- Boucher, R.C. Human airway ion transport. Part two. Am. J. Respir. Crit. Care Med. 1994, 150, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Quinton, P.M. Both ways at once: Keeping small airways clean. Physiology 2017, 32, 380–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamsuddin, A.K.; Quinton, P.M. Concurrent absorption and secretion of airway surface liquids and bicarbonate secretion in human brachioles. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L953–L960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunzelmann, K.; Schreiber, R.; Hadorn, H.B. Bicarbonate in cystic fibrosis. J. Cyst. Fibros. 2017, 16, 653–662. [Google Scholar] [CrossRef] [Green Version]
- Thornell, I.M.; Li, X.P.; Tang, X.X.; Brommel, C.M.; Karp, P.H.; Welsh, M.J.; Zabner, J. Nominal carbonic anhydrase activity minimizes airway-surface liquid pH changes during breathing. Physiol. Rep. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Alexandrou, D.; Walters, D.V. The role of Cl− in the regulation of ion and liquid transport in the intact alveolus during β-adrenergic stimulation. J. Exp. Physiol. 2013, 98, 576–584. [Google Scholar] [CrossRef]
- Cantiello, H.F.; Prat, A.G.; Reisin, I.L.; Ercole, L.B.; Abraham, E.H.; Amara, J.F.; Gregory, R.J.; Ausiello, D.A. External ATP and its analogs activate the cystic-fibrosis transmembrane conductance regulator by a cyclic amp-independent mechanism. J. Biol. Chem. 1994, 269, 11224–11232. [Google Scholar]
- Lazarowski, E.R.; Tarran, R.; Grubb, B.R.; van Heusden, C.A.; Okada, S.; Boucher, R.C. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J. Biol. Chem. 2004, 279, 36855–36864. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.W.; Chen, J.H.; Hughes, L.K.; Li, H.Y.; Sheppard, D.N. The Physiology and Pharmacology of the CFTR Cl- Channel. In Chloride Movements across Cellular Membranes, 1st ed.; Pursch, M., Ed.; Elsevier Science: San Diego, CA, USA, 2007; Volume 38, pp. 109–143. [Google Scholar] [CrossRef]
- Tang, L.; Fatehi, M.; Linsdell, P. Mechanism of direct bicarbonate transport by the CFTR anion channel. J. Cyst. Fibros. 2009, 8, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Sandefur, C.I.; Boucher, R.C.; Elston, T.C. Mathematical model reveals role of nucleotide signaling in airway surface liquid homeostasis and its dysregulation in cystic fibrosis. Proc. Natl. Acad. Sci. USA 2017, 114, E7272–E7281. [Google Scholar] [CrossRef]
- Wei, L.; Vankeerberghen, A.; Cuppens, H.; Eggermont, J.; Cassiman, J.J.; Droogmans, G.; Nilius, B. Interaction between calcium-activated chloride channels and the cystic fibrosis transmembrane conductance regulator. Pflügers Arch. 1999, 438, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Nam, J.H.; Park, H.W.; Oh, U.; Yoon, J.H.; Lee, M.G. Dynamic modulation of ANO1/TMEM16A HCO3- permeability by Ca2+/calmodulin. Proc. Natl. Acad. Sci. USA 2013, 110, 360–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Ion | KHS1 | KHS2 | KHS3 |
|---|---|---|---|
| Na+ | 141.8 | 24.8 | 141.8 |
| Cl− | 129.1 | 129.1 | 10.0 |
| K+ | 5.9 | 5.9 | 5.9 |
| Mg2+ | 1.2 | 1.2 | 1.2 |
| Ca2+ | 2.5 | 2.5 | 2.5 |
| HCO3− | 24.8 | 24.8 | 24.8 |
| H2PO4− | 1.2 | 1.2 | 1.2 |
| Choline | - | 117.0 | - |
| Gluconate | - | - | 119.1 |
| Glucose | 11.1 | 11.1 | 11.1 |
| Buffer in the Chamber | Fraction of Transported Chamber Volume Fluid (%) | |||
|---|---|---|---|---|
| Apical | Basolateral | Control | Amiloride | DIDS+Glibenclamide |
| KHS2 | KHS1 | 25.9 | 23.1 | 17.1 |
| KHS1 | KHS2 | 25.0 | 22.5 | 18.6 |
| KHS3 | KHS1 | 24.2 | 17.5 | 7.9 |
| KHS1 | KHS3 | 22.5 | 16.9 | 14.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zajac, M.; Lewenstam, A.; Bednarczyk, P.; Dolowy, K. Measurement of Multi Ion Transport through Human Bronchial Epithelial Cell Line Provides an Insight into the Mechanism of Defective Water Transport in Cystic Fibrosis. Membranes 2020, 10, 43. https://doi.org/10.3390/membranes10030043
Zajac M, Lewenstam A, Bednarczyk P, Dolowy K. Measurement of Multi Ion Transport through Human Bronchial Epithelial Cell Line Provides an Insight into the Mechanism of Defective Water Transport in Cystic Fibrosis. Membranes. 2020; 10(3):43. https://doi.org/10.3390/membranes10030043
Chicago/Turabian StyleZajac, Miroslaw, Andrzej Lewenstam, Piotr Bednarczyk, and Krzysztof Dolowy. 2020. "Measurement of Multi Ion Transport through Human Bronchial Epithelial Cell Line Provides an Insight into the Mechanism of Defective Water Transport in Cystic Fibrosis" Membranes 10, no. 3: 43. https://doi.org/10.3390/membranes10030043
APA StyleZajac, M., Lewenstam, A., Bednarczyk, P., & Dolowy, K. (2020). Measurement of Multi Ion Transport through Human Bronchial Epithelial Cell Line Provides an Insight into the Mechanism of Defective Water Transport in Cystic Fibrosis. Membranes, 10(3), 43. https://doi.org/10.3390/membranes10030043