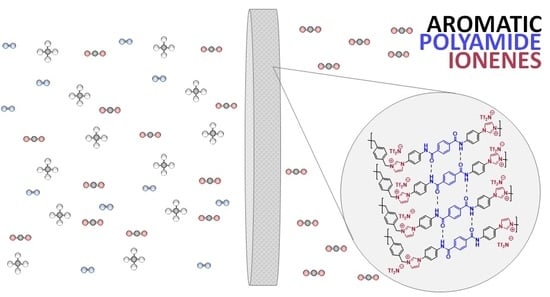
Synthesis and Performance of Aromatic Polyamide Ionenes as Gas Separation Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis of Bis-Imidazole Monomers Containing Amide Functionality
2.3.1. Synthesis of 4-(1H-imidazol-1-yl)aniline “I4A”, 3-(1H-imidazol-1-yl)aniline “I3A”, and 2-(1H-imidazol-1-yl)aniline “I2A”
2.3.2. Synthesis of Bis-Imidazole Amide Monomers
2.4. Synthesis of PA-Ionene Isomers
2.5. Gas Separation Measurements
3. Results & Discussion
3.1. Characterization of Materials
3.1.1. Structural Characterizations
3.1.2. Thermal Characterizations
3.2. Membrane Fabrication
3.3. Gas Permeation Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chiappetta, G.; Clarizia, G.; Drioli, E. Design of an integrated membrane system for a high level hydrogen purification. Chem. Eng. J. 2006, 124, 29–40. [Google Scholar] [CrossRef]
- Bernardo, P. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Koros, W.J.; Fleming, G.K. Membrane-based gas separation. J. Membr. Sci. 1993, 83, 1–80. [Google Scholar] [CrossRef]
- Koros, W.J. Polyamides and Polypyrrolones for Fluid Separation Membranes. U.S. Patent 5,262,056, 16 November 1993. [Google Scholar]
- Qiu, W.; Xu, L.; Chen, C.-C.; Paul, D.R.; Koros, W.J. Gas separation performance of 6fda-based polyimides with different chemical structures. Polymer 2013, 54, 6226–6235. [Google Scholar] [CrossRef]
- O’Harra, K.E.; Kammakakam, I.; Devriese, E.M.; Noll, D.M.; Bara, J.E.; Jackson, E.M. Synthesis and performance of 6fda-based polyimide-ionenes and composites with ionic liquids as gas separation membranes. Membranes 2019, 9, 79. [Google Scholar]
- Xiao, Y.; Low, B.T.; Hosseini, S.S.; Chung, T.S.; Paul, D.R. The strategies of molecular architecture and modification of polyimide-based membranes for co2 removal from natural gas—A review. Prog. Polym. Sci. 2009, 34, 561–580. [Google Scholar] [CrossRef]
- Mittenthal, M.S.; Flowers, B.S.; Bara, J.E.; Whitley, J.W.; Spear, S.K.; Roveda, J.D.; Wallace, D.A.; Shannon, M.S.; Holler, R.; Martens, R.; et al. Ionic polyimides: Hybrid polymer architectures and composites with ionic liquids for advanced gas separation membranes. Ind. Eng. Chem. Res. 2017, 56, 5055–5069. [Google Scholar] [CrossRef]
- Zhuang, Y.; Seong, J.G.; Lee, Y.M. Polyimides containing aliphatic/alicyclic segments in the main chains. Prog. Polym. Sci. 2019, 92, 35–88. [Google Scholar] [CrossRef]
- Kammakakam, I.; Nam, S.; Kim, T.-H. Ionic group-mediated crosslinked polyimide membranes for enhanced co2 separation. RSC Adv. 2015, 5, 69907–69914. [Google Scholar] [CrossRef]
- McKeown, N.B. Polymers of intrinsic microporosity. ISRN Mater. Sci. 2012, 2012, 513986. [Google Scholar] [CrossRef] [Green Version]
- Budd, P.; McKeown, N.; Ghanem, B.; Msayib, K.; Fritsch, D.; Starannikova, L.; Belov, N.; Sanfirova, O.; Yampolskii, Y.; Shantarovich, V. Gas permeation parameters and other physicochemical properties of a polymer of intrinsic microporosity: Polybenzodioxane pim-1. J. Membr. Sci. 2008, 325, 851–860. [Google Scholar] [CrossRef]
- Smith, S.J.D.; Hou, R.; Lau, C.H.; Konstas, K.; Kitchin, M.; Dong, G.; Lee, J.; Lee, W.H.; Seong, J.G.; Lee, Y.M.; et al. Highly permeable thermally rearranged mixed matrix membranes (tr-mmm). J. Membr. Sci. 2019, 585, 260–270. [Google Scholar] [CrossRef]
- Li, S.; Jo, H.J.; Han, S.H.; Park, C.H.; Kim, S.; Budd, P.M.; Lee, Y.M. Mechanically robust thermally rearranged (tr) polymer membranes with spirobisindane for gas separation. J. Membr. Sci. 2013, 434, 137–147. [Google Scholar] [CrossRef]
- Shamsipur, H.; Dawood, B.A.; Budd, P.M.; Bernardo, P.; Clarizia, G.; Jansen, J.C. Thermally rearrangeable pim-polyimides for gas separation membranes. Macromolecules 2014, 47, 5595–5606. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.M. Thermally rearranged (tr) polymer membranes with nanoengineered cavities tuned for co2 separation. J. Nanopart. Res. 2012, 14, 949. [Google Scholar] [CrossRef]
- Kammakakam, I.; O’Harra, K.E.; Dennis, G.P.; Jackson, E.M.; Bara, J.E. Self-healing imidazolium-based ionene-polyamide membranes: An experimental study on physical and gas transport properties. Polym. Int. 2019, 68, 1123–1129. [Google Scholar] [CrossRef]
- Hoehn, H.H. Aromatic polyamide membranes. Mater. Sci. Synth. Membr. 1985, 269, 81–98. [Google Scholar]
- Kirsh, Y.E. Ion-exchange, pervaporation and reverse-osmosis membranes based on aromatic polyamides: Structure, selectivity, permeability and mechanisms of function. Ross. Khimicheskii Zhurnal 1998, 42, 117–129. [Google Scholar]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Tome, L.C.; Marrucho, I.M. Ionic liquid-based materials: A platform to design engineered CO2 separation membranes. Chem. Soc. Rev. 2016, 45, 2785–2824. [Google Scholar] [CrossRef]
- Bara, J.E.; O’Harra, K.E.; Durbin, M.M.; Dennis, G.P.; Jackson, E.M.; Thomas, B.; Odutola, J.A. Synthesis and characterization of ionene-polyamide materials as candidates for new gas separation membranes. MRS Adv. 2018, 3, 3091–3102. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.C.; Shimamura, K.; Mishra, A.; Shimojo, F.; Nakano, A.; Kalia, R.K.; Vashishta, P.; Branicio, P.S. Hydrogen bond preserving stress release mechanism is key to the resilience of aramid fibers. J. Phys. Chem. B 2019, 123, 9719–9723. [Google Scholar] [CrossRef] [PubMed]
- Murthy, N.S. Hydrogen bonding, mobility, and structural transitions in aliphatic polyamides. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1763–1782. [Google Scholar] [CrossRef]
- Ji, C.; Xue, S.; Tang, Y.-J.; Ma, X.-H.; Xu, Z.-L. Polyamide membranes with net-like nanostructures induced by different charged mofs for elevated nanofiltration. ACS Appl. Polym. Mater. 2019. [Google Scholar] [CrossRef]
- Guo, Y.-S.; Mi, Y.-F.; Ji, Y.-L.; An, Q.-F.; Gao, C.-J. One-step surface grafting method for preparing zwitterionic nanofiltation membrane via in situ introduction of initiator in interfacial polymerization. ACS Appl. Polym. Mater. 2019, 1, 1022–1033. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Kang, Y.; Liu, Y.; Mi, B. Grafting polyzwitterions onto polyamide by click chemistry and nucleophilic substitution on nitrogen: A novel approach to enhance membrane fouling resistance. J. Membr. Sci. 2014, 449, 50–57. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Thérien-Aubin, H.; Ben-Sasson, M.; Ober, C.K.; Nielsen, M.; Elimelech, M. Control of biofouling on reverse osmosis polyamide membranes modified with biocidal nanoparticles and antifouling polymer brushes. J. Mater. Chem. B 2014, 2, 1724–1732. [Google Scholar] [CrossRef]
- Perreault, F.; Tousley, M.E.; Elimelech, M. Thin-film composite polyamide membranes functionalized with biocidal graphene oxide nanosheets. Environ. Sci. Technol. Lett. 2013, 1, 71–76. [Google Scholar] [CrossRef]
- Ma, W.; Chen, T.; Nanni, S.; Yang, L.; Ye, Z.; Rahaman, M.S. Zwitterion-functionalized graphene oxide incorporated polyamide membranes with improved antifouling properties. Langmuir 2019, 35, 1513–1525. [Google Scholar] [CrossRef]
- Tiraferri, A.; Vecitis, C.D.; Elimelech, M. Covalent binding of single-walled carbon nanotubes to polyamide membranes for antimicrobial surface properties. ACS Appl. Mater. Interfaces 2011, 3, 2869–2877. [Google Scholar] [CrossRef]
- Duong, P.H.H.; Hong, P.-Y.; Musteata, V.; Peinemann, K.V.; Nunes, S.P. Thin film polyamide membranes with photoresponsive antibacterial activity. ChemistrySelect 2017, 2, 6612–6616. [Google Scholar] [CrossRef]
- Gohil, J.M.; Ray, P. A review on semi-aromatic polyamide tfc membranes prepared by interfacial polymerization: Potential for water treatment and desalination. Sep. Purif. Technol. 2017, 181, 159–182. [Google Scholar] [CrossRef]
- Kong, C.; Kanezashi, M.; Yamomoto, T.; Shintani, T.; Tsuru, T. Controlled synthesis of high performance polyamide membrane with thin dense layer for water desalination. J. Membr. Sci. 2010, 362, 76–80. [Google Scholar] [CrossRef]
- Morisato, A.; Ghosal, K.; Freeman, B.D.; Chern, R.T.; Alvarez, J.C.; de la Campa, J.G.; Lozano, A.n.E.; de Abajo, J. Gas separation properties of aromatic polyamides containing hexafluoroisopropylidene groups. J. Membr. Sci. 1995, 104, 231–241. [Google Scholar] [CrossRef]
- Ren, L.; Liu, J. Synthesis and gas transport properties of polyamide membranes containing pdms groups. RSC Adv. 2019, 9, 9737–9744. [Google Scholar] [CrossRef] [Green Version]
- Rabiee, H.; Ghadimi, A.; Mohammadi, T. Gas transport properties of reverse-selective poly(ether-b-amide6)/[emim][bf4] gel membranes for co2/light gases separation. J. Membr. Sci. 2015, 476, 286–302. [Google Scholar] [CrossRef]
- González-Díaz, M.O.; Sulub-Sulub, R.; Herrera-Kao, W.; Vázquez-Torres, H.; Zolotukhin, M.G.; Aguilar-Vega, M. Enhanced gas transport performance of polyamide membranes by postpolymerization modification. Ind. Eng. Chem. Res. 2018, 57, 8989–8996. [Google Scholar] [CrossRef]
- Bisoi, S.; Mandal, A.K.; Singh, A.; Banerjee, S. Gas separation properties of troeger’s base-bridged polyamides. e-Polymers 2017, 17, 283–293. [Google Scholar] [CrossRef]
- Amoli-Diva, M.; Irani, E.; Pourghazi, K. Photocatalytic filtration reactors equipped with bi-plasmonic nanocomposite/poly acrylic acid-modified polyamide membranes for industrial wastewater treatment. Sep. Purif. Technol. 2020, 236, 116257. [Google Scholar] [CrossRef]
- Gu, J.-E.; Lee, J.S.; Park, S.-H.; Kim, I.T.; Chan, E.P.; Kwon, Y.-N.; Lee, J.-H. Tailoring interlayer structure of molecular layer-by-layer assembled polyamide membranes for high separation performance. Appl. Surf. Sci. 2015, 356, 659–667. [Google Scholar] [CrossRef]
- Rajesh, S.; Zhao, Y.; Fong, H.; Menkhaus, T.J. Nanofiber multilayer membranes with tailored nanochannels prepared by molecular layer-by-layer assembly for high throughput separation. J. Mater. Chem. A 2017, 5, 4616–4628. [Google Scholar] [CrossRef]
- Yuan, S.; Strobbe, D.; Li, X.; Kruth, J.-P.; Van Puyvelde, P.; Van der Bruggen, B. 3d printed chemically and mechanically robust membrane by selective laser sintering for separation of oil/water and immiscible organic mixtures. Chem. Eng. J. 2020, 385, 123816. [Google Scholar] [CrossRef]
- Chowdhury, M.R.; Steffes, J.; Huey, B.D.; McCutcheon, J.R. 3d printed polyamide membranes for desalination. Science 2018, 361, 682–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, G.-Y.; Krantz, W.B. Formation and characterization of polyamide membranes via interfacial polymerization. J. Membr. Sci. 1994, 93, 175–192. [Google Scholar] [CrossRef]
- Sridhar, S.; Smitha, B.; Mayor, S.; Prathab, B.; Aminabhavi, T.M. Gas permeation properties of polyamide membrane prepared by interfacial polymerization. J. Mater. Sci. 2007, 42, 9392–9401. [Google Scholar] [CrossRef]
- Kammakakam, I.; O’Harra, K.E.; Bara, J.E.; Jackson, E.M. Design and synthesis of imidazolium-mediated tröger’s base-containing ionene polymers for advanced co2 separation membranes. ACS Omega 2019, 4, 3439–3448. [Google Scholar] [CrossRef] [Green Version]
- Bara, J.E.; O’Harra, K.E. Recent advances in the design of ionenes: Toward convergence with high-performance polymers. Macromol. Chem. Phys. 2019, 220, 1900078. [Google Scholar] [CrossRef]
- Matthews, R.P.; Welton, T.; Hunt, P.A. Hydrogen bonding and pi-pi interactions in imidazolium-chloride ionic liquid clusters. Phys. Chem. Chem. Phys. 2015, 17, 14437–14453. [Google Scholar] [CrossRef]
- Li, P.; Paul, D.R.; Chung, T.-S. High performance membranes based on ionic liquid polymers for co2 separation from the flue gas. Green Chem. 2012, 14, 1052–1063. [Google Scholar] [CrossRef]
- Kammakakam, I.; Kim, H.W.; Nam, S.; Park, H.B.; Kim, T.-H. Alkyl imidazolium-functionalized cardo-based poly(ether ketone)s as novel polymer membranes for o2/n2 and co2/n2 separations. Polymer 2013, 54, 3534–3541. [Google Scholar] [CrossRef]
- Gómez-Valdemoro, A.; San-José, N.; García, F.C.; De La Peña, J.L.; Serna, F.; García, J.M. Novel aromatic polyamides with main chain and pendant 1,2,4-triazole moieties and their application to the extraction/elimination of mercury cations from aqueous media. Polym. Chem. 2010, 1, 1291–1301. [Google Scholar] [CrossRef]
- Halasa, A.F.; Wathen, G.D.; Hsu, W.L.; Matrana, B.A.; Massie, J.M. Relationship between interchain spacing of amorphous polymers and blend miscibility as determined by wide-angle x-ray scattering. J. Appl. Polym. Sci. 1991, 43, 183–190. [Google Scholar] [CrossRef]





| ID | PA-Ionene | Bis-Imidazole | Amount | Linkage | Amount | LiTf2N | Product Yield | Yield |
|---|---|---|---|---|---|---|---|---|
| 1 | [TC I4A pX][Tf2N] | TC I4A | 10.1 g | 22.6 mmol | pDCX | 3.95 g | 22.6 mmol | 16.2 g | 21.5 g | 86% |
| 2 | [IC I3A mX][Tf2N] | IC I3A | 8.00 g | 17.8 mmol | mDCX | 2.50 g | 5.57 mmol | 12.8 g | 14.1 g | 71% |
| 3 | [TC I3A mX][Tf2N] | TC I3A | 2.50 g | 5.57 mmol | mDCX | 0.98 g | 5.57 mmol | 4.00 g | 3.89g | 62% |
| 4 | [TC I3A pX][Tf2N] | TC I3A | 2.50 g | 5.57 mmol | pDCX | 0.98 g | 5.57 mmol | 4.00 g | 4.31 g | 70% |
| 5 | [IC I4A pX][Tf2N] | IC I4A | 2.00 g | 4.46 mmol | pDCX | 0.78 g | 4.46 mmol | 3.20 g | 2.76 g | 56% |
| 6 | [IC I4A mX][Tf2N] | IC I4A | 2.00 g | 4.46 mmol | mDCX | 0.78 g | 4.46 mmol | 3.20 g | 2.41 g | 49% |
| 7 | [TC I2A oX][Tf2N] | TC I2A | 1.50 g | 3.35 mmol | oDCX | 0.58 g | 3.35 mmol | 2.40 g | 2.84 g | 76% |
| ID | PA-Ionene | IL (Equivalents) | Tg (°C) | d-Spacing (Å) | Density (g/cm3) | MN (kDa) |
|---|---|---|---|---|---|---|
| 1 | [TC I4A pX][Tf2N] | Neat | 136.1 | 4.07 | 1.517 | 60.4 |
| 1 + IL | [Bnmim][Tf2N] (1) | - | 4.26 | 1.575 | * | |
| 2 | [IC I3A mX][Tf2N] | Neat | 133.5 | 4.35 | 1.443 | 62.5 |
| 2 + IL | [Bnmim][Tf2N] (1) | - | 4.55 | 1.259 | * | |
| 3 | [TC I3A mX][Tf2N] | Neat | 54.5 | 4.36 | 1.388 | 59.9 |
| 4 | [TC I3A pX][Tf2N] | Neat | 74.7 | 4.39 | 1.427 | 75.4 |
| 5 | [IC I4A pX][Tf2N] | Neat | 112.0 | 4.97 | 1.505 | 66.4 |
| 6 | [IC I4A mX][Tf2N] | Neat | 115.7 | 5.09 | 1.462 | 76.1 |
| 7 | [TC I2A oX][Tf2N] | Neat | 76.4 | 4.47 | 1.577 | 117.7 |
| ID | PA-Ionene | Permeability and Selectivity Data a,b | |||||
|---|---|---|---|---|---|---|---|
| PCO2 | PN2 | PCH4 | αCO2/N2 | αCO2/CH4 | |||
| 1 | [TC I4A pX][Tf2N] | Neat | 11.00 ± 0.02 | 0.34 ± 0.02 | 0.23 ± 0.01 | 32.6 ± 1.9 | 46.9 ± 2.0 |
| 1 + IL | [Bnmim][Tf2N] | 0.403 ± 0.06 | 0.112 ± 0.002 | 0.087 ± 0.031 | 3.6 ± 0.5 | 4.6 ± 1.8 | |
| 3 | [TC I3A mX][Tf2N] | Neat | 3.91 ± 0.04 | 0.096 ± 0.004 | 0.122 ± 0.003 | 40.9 ± 1.8 | 30.5 ± 0.08 |
| 4 | [TC I3A pX][Tf2N] | Neat | 2.46 ± 0.03 | 0.082 ± 0.005 | 0.103 ± 0.003 | 30.2 ± 1.4 | 23.9 ± 0.08 |
| 5 | [IC I4A pX][Tf2N] | Neat | 1.69 ± 0.05 | 0.046 ± 0.004 | 0.039 ± 0.005 | 36.6 ± 3.4 | 43.3 ± 5.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Harra, K.E.; Kammakakam, I.; Noll, D.M.; Turflinger, E.M.; Dennis, G.P.; Jackson, E.M.; Bara, J.E. Synthesis and Performance of Aromatic Polyamide Ionenes as Gas Separation Membranes. Membranes 2020, 10, 51. https://doi.org/10.3390/membranes10030051
O’Harra KE, Kammakakam I, Noll DM, Turflinger EM, Dennis GP, Jackson EM, Bara JE. Synthesis and Performance of Aromatic Polyamide Ionenes as Gas Separation Membranes. Membranes. 2020; 10(3):51. https://doi.org/10.3390/membranes10030051
Chicago/Turabian StyleO’Harra, Kathryn E., Irshad Kammakakam, Danielle M. Noll, Erika M. Turflinger, Grayson P. Dennis, Enrique M. Jackson, and Jason E. Bara. 2020. "Synthesis and Performance of Aromatic Polyamide Ionenes as Gas Separation Membranes" Membranes 10, no. 3: 51. https://doi.org/10.3390/membranes10030051
APA StyleO’Harra, K. E., Kammakakam, I., Noll, D. M., Turflinger, E. M., Dennis, G. P., Jackson, E. M., & Bara, J. E. (2020). Synthesis and Performance of Aromatic Polyamide Ionenes as Gas Separation Membranes. Membranes, 10(3), 51. https://doi.org/10.3390/membranes10030051