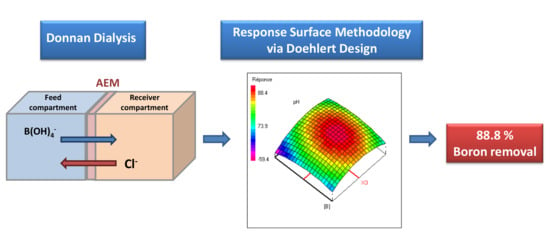
Response Surface Methodology for Boron Removal by Donnan Dialysis: Doehlert Experimental Design
Abstract
:1. Introduction
2. Experimental
2.1. Membranes
2.2. Donnan Dialysis (DD)
2.3. Optimization of the Removal of Boron by Donnan Dialysis
3. Results and Discussion
3.1. The Preliminary Study
3.1.1. Effect of pH in the Feed Compartment
3.1.2. Effect of Chloride Concentration in the Receiver Compartment
3.1.3. Effect of Boron Concentration in the Feed Compartment
3.1.4. Membrane Choice
3.2. Full Factorial Design
3.3. Response Surface Methodology
3.3.1. Doehlert Design
3.3.2. Experimental Field
3.3.3. Modelling of Donnan Dialysis
3.3.4. Validation of Models
3.3.5. Optimization by Response Surface Methodology as Doehlert Design
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brdar-Jokanovic, M. Boron Toxicity and Deficiency in Agricultural Plants. Int. J. Mol. Sci. 2020, 21, 1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, A.; Khan, M.K.; Hakki, E.E.; Gezgin, S.; Hamurcu, M. Combined Boron Toxicity and Salinity Stress—An Insight into Its Interaction in Plants. Plants 2019, 8, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guler, E.; Kaya, C.; Kabay, N.; Arda, M. Boron removal from seawater: State of the art review. Desalination 2015, 356, 85–93. [Google Scholar] [CrossRef]
- Chruszcz-Lipska, K.; Winid, B.; Madalska, G.A.; Macuda, J.; Lukanko, L. High Content of Boron in Curative Water: From the Spa to Industrial Recovery of Borates? (Poland as a Case Study). Minerals 2021, 11, 8. [Google Scholar] [CrossRef]
- Lyu, J.; Zhang, N.; Liu, H.; Zeng, Z.; Zhang, J.; Bai, P.; Guo, X. Adsorptive removal of boron by zeolitic imidazolate framework: Kinetics, isotherms, thermodynamics, mechanism and recycling. Sep. Purif. Technol. 2017, 187, 67–75. [Google Scholar] [CrossRef]
- Ting, T.M.; Nasef, M.M.; Aravindan, D.; Rosslan, I.F.N.; Ruslan, N. Selective removal of boron from industrial wastewater containing high concentration of ammonia by radiation grafted fibrous adsorbent in fixed bed column. J. Environ. Chem. Eng. 2021, 9, 104993. [Google Scholar] [CrossRef]
- Yilmaz, A.; Boncukcuoğlu, R.; Kocakerim, M. A quantitative comparison between electrocoagulation and chemical coagulation for boron removal from boron-containing solution. J. Hazard. Mater. 2007, 149, 475–481. [Google Scholar] [CrossRef]
- Chen, M.; Dollar, O.; Shafer-Peltier, K.; Randtke, S.; Waseem, S.; Peltier, E. Boron removal by electrocoagulation: Removal mechanism, adsorption models and factors influencing removal. Water Res. 2020, 170, 115362. [Google Scholar] [CrossRef]
- Turek, M.; Dydo, P.; Bandura-Zalska, B. Boron removal from dual-staged seawater nanofiltration permeate by electrodialysis. Desalin. Water Treat. 2009, 10, 60–63. [Google Scholar] [CrossRef]
- Guesmi, F.; Louati, I.; Hannachi, C.; Hamrouni, B. Optimization of boron removal from water by electrodialysis using response surface methodology. Water Sci. Technol. 2020, 81, 293–300. [Google Scholar] [CrossRef]
- Ali, Z.; Al Sunbul, Y.; Pacheco, F.; Ogieglo, W.; Wang, Y.; Genduso, G.; Pinnau, I. Defect-free highly selective polyamide thin-film composite membranes for desalination and boron removal. J. Membr. Sci. 2019, 578, 85–94. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Song, X.; Zhou, Y.; Shen, H.; Cao, X.; Zhang, P.; Gao, C. High boron removal polyamide reverse osmosis membranes by swelling induced embedding of a sulfonyl molecular plug. J. Membr. Sci. 2020, 597, 117716. [Google Scholar] [CrossRef]
- Kumar, R.; Ahmed, M.; Ok, S.; Garudachari, B.; Thomas, J.P. Boron selective thin film composite nanofiltration membrane fabricated via a self-assembled trimesic acid layer at a liquid–liquid interface on an ultrafiltration support. New J. Chem. 2019, 43, 3874–3883. [Google Scholar] [CrossRef]
- Lan, N.; Wang, K.Y.; Weber, M.; Maletzko, C.; Chung, T.S. Investigation of novel molecularly tunable thin-film nanocomposite nanofiltration hollow fiber membranes for boron removal. J. Membr. Sci. 2021, 620, 118887. [Google Scholar] [CrossRef]
- Alharati, A.; Swesi, Y.; Fiaty, K.; Charcosset, C. Boron removal in water using a hybrid membrane process of ion exchange resin and microfiltration without continuous resin addition. J. Water Process. Eng. 2017, 17, 32–39. [Google Scholar] [CrossRef]
- Hussain, A.; Sharma, R.; Minier-Matar, J.; Hirani, Z.; Adhama, S. Application of emerging ion exchange resin for boron removal from saline groundwater. J. Water Process. Eng. 2019, 32, 100906–100915. [Google Scholar] [CrossRef]
- Eryildiz, B.; Yuksekdag, A.; Korkut, S.; Koyuncu, İ. Performance evaluation of boron removal from wastewater containing high boron content according to operating parameters by air gap membrane distillation. Environ. Technol. Innov. 2021, 22, 101493. [Google Scholar] [CrossRef]
- Turek, M.; Bandura-Zalska, B.; Dydo, P. Boron removal by Donnan dialysis. Desalin. Water Treat. 2009, 10, 53–59. [Google Scholar] [CrossRef]
- Bryjak, M.; Duraj, I. Anion-exchange membranes for separation of borates by Donnan dialysis. Desalination 2013, 310, 39–42. [Google Scholar] [CrossRef]
- DiNunzio, J.E.; Jubara, M. Donnan Dialysis Preconcentration for Ion Chromatography. Anal. Chem. 1983, 55, 1013–1016. [Google Scholar] [CrossRef]
- Sato, K. Effects of the feed solution concentration on the separation degree in Donnan dialysis for binary systems of amino acids. J. Membr. Sci. 2002, 196, 211–220. [Google Scholar] [CrossRef]
- Seneviratne, J.; Holmstrom, S.D.; Cox, J.A. Donnan Dialysis Preconcentration Coupled with Ion Chromatography and Electrocatalytic Oxidation for the Determination of Cyanide. Talanta 2000, 52, 1025–1031. [Google Scholar] [CrossRef]
- Ben Hamouda, S.; Touati, K.; Ben Amor, M. Donnan dialysis as membrane process for nitrate removal from drinking water: Membrane structure effect. Arab. J. Chem. 2017, 10, S287–S292. [Google Scholar] [CrossRef] [Green Version]
- Marzouk Trifi, I.; Trifi, B.; Djamel, A.; Hamrouni, B. Simultaneous removal of nitrate and nitrite by Donnan Dialysis. Environ. Eng. Manag. J. 2020, 20, 973–983. [Google Scholar]
- Dieye, A.; Larchet, C.; Auclair, B.; Mar-Diop, C. Elimination des fluorures par la dialyse ionique croisée. Eur. Polym. J. 1998, 34, 67–75. [Google Scholar] [CrossRef]
- Hichour, M.; Persin, F.; Sandeaux, J.; Gavach, C. Fluoride removal from waters by Donnan dialysis. Sep. Purif. Technol. 2000, 18, 1–11. [Google Scholar] [CrossRef]
- Hichour, M.; Persin, F.; Molenat, J.; Sandeaux, J.; Gavach, C. Fluoride removal from diluted solutions by donnan dialysis with anion exchange membranes. Desalination 1999, 122, 53–62. [Google Scholar] [CrossRef]
- Marzouk, I.; Dammak, L.; Chaabane, L.; Hamrouni, B. Optimization of chromium (VI) removal by Donnan dialysis. Am. J. Anal. Chem. 2013, 4, 306–313. [Google Scholar] [CrossRef] [Green Version]
- Marzouk, I.; Chaabane, L.; Dammak, L.; Hamrouni, B. Application of Donnan dialysis coupled to adsorption onto activated alumina for chromium (VI) removal. Am. J. Anal. Chem. 2013, 4, 420–425. [Google Scholar] [CrossRef] [Green Version]
- Marzouk Trifi, I.; Trifi, B.; Ben Ayed, S.; Hamrouni, B. Removal of phosphate by Donnan dialysis coupled to adsorption onto alginate calcium beads. Water Sci. Technol. 2019, 80, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Rangaiah, G.P.; Feng, Z.; Hoadley, A.F. Multi-Objective Optimization Applications in Chemical Process Engineering: Tutorial and Review. Processes 2020, 8, 508. [Google Scholar] [CrossRef]
- French Standard NF X 45-200, Membranes Polymers Échangeuse d’Ions; AFNOR: Paris, France, 1995.
- Strathmann, H. Ion-Exchange Membrane Separation Processes, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Nouri, S.; Dammak, L.; Bulvestre, G.; Auclair, B. Studies of the crossed ionic fluxes through a cation-exchange membrane in the case of Donnan dialysis. Desalination 2002, 148, 383–388. [Google Scholar] [CrossRef]
- Mokrani, S.; Dammak, L.; Bulvestre, G.; Auclair, B. Experimental and theoretical studies of the crossed ionic fluxes through a cation-exchange membrane. J. Membr. Sci. 2002, 199, 147–160. [Google Scholar] [CrossRef]
- Wisniewski, J.; Rozanska, A. Donnan dialysis with anion exchange membranes as a pretreatment step before electrodialytic desalination. Desalination 2006, 191, 210–218. [Google Scholar] [CrossRef]
- Rozanska, A.; Wiśniewski, J.; Winnicki, T. Donnan dialysis with anion-exchange membranes in a water desalination system. Desalination 2006, 198, 236–246. [Google Scholar] [CrossRef]
- Rodier, J. L’analyse de l’eau, 9th ed.; Edition Dunod: Paris, France, 2009. [Google Scholar]
- Zohdi, N.; Mahdavi, F.; Abdullah, L.C.; Choong, T.S.Y. Removal of boron from aqueous solution using magnetic carbon nanotube improved with tartaric acid. J. Environ. Health Sci. Eng. 2014, 12, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayyildiz, H.F.; Kara, H. Boron removal by ion exchange membranes. Desalination 2005, 180, 99–108. [Google Scholar] [CrossRef]
- Veerman, J. The Effect of the NaCl Bulk Concentration on the Resistance of Ion Exchange Membranes—Measuring and Modeling. Energies 2020, 13, 1946. [Google Scholar] [CrossRef] [Green Version]
- Amang, D.N.; Alexandrova, S.; Schaetzel, P. Mass transfer characterization of Donnan dialysis in a bi-ionic chloride–nitrate system. Chem. Eng. J. 2004, 99, 69–76. [Google Scholar] [CrossRef]
- Wisniewski, J.; Rozanska, A.; Winnicki, T. Removal of troublesome anions from water by means of Donnan dialysis. Desalination 2005, 182, 339–346. [Google Scholar] [CrossRef]
- Tor, A.; Çengeloğlu, Y.; Ersöz, M.; Arslan, G. Transport of chromium through cation-exchange membranes by Donnan dialysis in the presence of some metals of different valences. Desalination 2004, 170, 151–159. [Google Scholar] [CrossRef]
- Akretche, D.; Kerdjoudj, H. Donnan dialysis of copper, gold and silver cyanides with various anion exchange membranes. Talanta 2000, 51, 281–289. [Google Scholar] [CrossRef]
- Hosni, N.; Zehani, K.; Djebali, K.; Mazaleyrat, F.; Bessais, L.; Maghraoui-Meherzi, H. Experimental design approach for the synthesis of 3D-CoFe2O4 nanoflowers thin films by low-cost process. Mater. Chem. Phys. 2020, 255, 123493–123503. [Google Scholar] [CrossRef]
- Nde Bup, D.; Abi, C.F.; Tenin, D.; Kapseu, C.; Tchiegang, C. Optimisation of the cooking process of sheanut kernels (vitellaria paradoxa gaertn.) using the Doehlert experimental design. Food. Bioproc. Technol. 2012, 5, 108–117. [Google Scholar] [CrossRef]







| Membranes | Ion-Exchange Capacity (meq/g) | Water Content (%) | Thickness (μm) |
|---|---|---|---|
| Neosepta® ACS | 1.85 | 18.9 | 150 |
| Neosepta® AFN | 3.00 | 47.8 | 120 |
| Factors | Symbol | Coded Symbol | Range and Level | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Initial concentration of boron (mg/L) | [B] | X1 | 25 | 100 |
| Concentration of counter-ions (mol/L) | [Cl−] | X2 | 0.1 | 0.5 |
| pH of the solution | pH | X3 | 10.5 | 12.5 |
| N° | [Cl−] | [B] | pH | [Cl−] | [B] | pH | YB(%)exp | YB(%)cal | Relative Difference (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 0.1 | 25 | 10.5 | 19.9 | 20.2 | 1.50 |
| 2 | +1 | −1 | −1 | 0.5 | 25 | 10.5 | 27.7 | 27.4 | 1.09 |
| 3 | −1 | +1 | −1 | 0.1 | 100 | 10.5 | 30.7 | 30.4 | 0.98 |
| 4 | +1 | +1 | −1 | 0.5 | 100 | 10.5 | 36.5 | 36.8 | 0.82 |
| 5 | −1 | −1 | +1 | 0.1 | 25 | 12.5 | 36.9 | 36.6 | 0.82 |
| 6 | +1 | −1 | +1 | 0.5 | 25 | 12.5 | 39.9 | 40.2 | 0.75 |
| 7 | −1 | +1 | +1 | 0.1 | 100 | 12.5 | 41.9 | 42.2 | 0.71 |
| 8 | +1 | +1 | +1 | 0.5 | 100 | 12.5 | 45.3 | 45.0 | 0.66 |
| Factors | Range and Levels | ||||||
|---|---|---|---|---|---|---|---|
| Coded Variable X1 | −1 | −0.5 | 0 | 0.5 | 1 | ||
| Concentration of Cl− (mol/L) | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | ||
| Coded Variable X2 | −0.866 | −0.577 | −0.287 | 0 | 0.287 | 0.577 | 0.866 |
| pH | 10.6 | 10.9 | 11.2 | 11.5 | 11.8 | 12.1 | 12.4 |
| Coded Variable X3 | −0.816 | 0 | 0.816 | ||||
| Concentration of Boron (mg/L) | 31 | 62 | 93 | ||||
| N° | X1 | X2 | X3 | [Cl−] | pH | [B] | Y(%)Exp | Y(%)Cal | Relative Difference (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.0 | 0.000 | 0.000 | 0.5 | 11.5 | 62 | 87.3 | 87.4 | 0.11 |
| 2 | −1.0 | 0.000 | 0.000 | 0.1 | 11.5 | 62 | 81.5 | 81.4 | 0.12 |
| 3 | 0.5 | 0.866 | 0.000 | 0.4 | 12.4 | 62 | 51.4 | 51.6 | 0.39 |
| 4 | −0.5 | −0.866 | 0.000 | 0.2 | 10.6 | 62 | 16.1 | 15.9 | 1.25 |
| 5 | 0.5 | −0.866 | 0.000 | 0.4 | 10.6 | 62 | 21.1 | 20.7 | 1.91 |
| 6 | −0.5 | 0.866 | 0.000 | 0.2 | 12.4 | 62 | 50.7 | 50.6 | 0.19 |
| 7 | 0.5 | 0.287 | 0.816 | 0.4 | 11.8 | 93 | 65.2 | 64.9 | 0.47 |
| 8 | −0.5 | −0.287 | −0.816 | 0.2 | 11.2 | 31 | 35.9 | 36.2 | 0.83 |
| 9 | 0.5 | −0.287 | −0.816 | 0.4 | 11.2 | 31 | 40.1 | 39.9 | 0.50 |
| 10 | 0.0 | 0.577 | −0.816 | 0.3 | 12.1 | 31 | 46.6 | 46.5 | 0.21 |
| 11 | −0.5 | 0.287 | 0.816 | 0.2 | 11.8 | 93 | 62.6 | 62.7 | 0.16 |
| 12 | 0.0 | −0.577 | 0.816 | 0.3 | 10.9 | 93 | 39.5 | 39.6 | 0.25 |
| 13 | 0.0 | 0.000 | 0.000 | 0.3 | 11.5 | 62 | 84.2 | 84.2 | 0.00 |
| 14 | 0.0 | 0.000 | 0.000 | 0.3 | 11.5 | 62 | 84.2 | 84.2 | 0.00 |
| 15 | 0.0 | 0.000 | 0.000 | 0.3 | 11.5 | 62 | 84.2 | 84.2 | 0.00 |
| Coefficients | p-Values | |
|---|---|---|
| b0 | 84.2 | 0.0001 |
| b1 | 3.01 | 0.0001 |
| b2 | 18.81 | 0.0001 |
| b3 | 9.12 | 0.0001 |
| b11 | 0.20 | 0.394 |
| b22 | −65.90 | 0.0001 |
| b33 | −37.40 | 0.0001 |
| b12 | −2.38 | 0.002 |
| b13 | −0.10 | 0.750 |
| b23 | −12.20 | 0.0001 |
| R2 | 0.9999 | |
| AED (%) | 0.425 | |
| Source Model | Degrees of Freedom | Sum of Square | Mean of Square | F-Value | Ftable (α = 5%) | p-Value |
|---|---|---|---|---|---|---|
| Regression | 9 | 7988.2 | 887.6 | 88,758.7 | 4.54 | 0.0001 |
| Residual | 5 | 389.1 | 129.7 | |||
| Total | 14 | 8377.4 |
| Reference | Composition of Feed and Receiver Compartment | Efficiency for Boron Removal (%) |
|---|---|---|
| [40] | Feed: 0.1 mol/L of B pH = 9.5 Receiver: 0.1 mol/L of Cl− | 0.1% removal with AHA membrane 0.1% removal with AMH membrane 0.5% removal with AFN membrane |
| [18] | Feed: 75 mg/L of B pH = 11.1 Receiver: 1.0 mol/L of OH− | 0.8% removal with AMX membrane |
| [19] | Feed: 20 mg/L of B pH = 9.5 Receiver: 1 mol/L of Cl− | 30% removal by PEI−2 membrane 40% removal by PEI-3 membrane |
| Our work | Feed: 66 mg/L of B pH = 11.5 Receiver: 0.5 mol/L of Cl− | 88.8% removal by AFN membrane |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifi, I.M.; Chaabane, L.; Dammak, L.; Baklouti, L.; Hamrouni, B. Response Surface Methodology for Boron Removal by Donnan Dialysis: Doehlert Experimental Design. Membranes 2021, 11, 731. https://doi.org/10.3390/membranes11100731
Trifi IM, Chaabane L, Dammak L, Baklouti L, Hamrouni B. Response Surface Methodology for Boron Removal by Donnan Dialysis: Doehlert Experimental Design. Membranes. 2021; 11(10):731. https://doi.org/10.3390/membranes11100731
Chicago/Turabian StyleTrifi, Ikhlass Marzouk, Lobna Chaabane, Lasâad Dammak, Lassaad Baklouti, and Béchir Hamrouni. 2021. "Response Surface Methodology for Boron Removal by Donnan Dialysis: Doehlert Experimental Design" Membranes 11, no. 10: 731. https://doi.org/10.3390/membranes11100731
APA StyleTrifi, I. M., Chaabane, L., Dammak, L., Baklouti, L., & Hamrouni, B. (2021). Response Surface Methodology for Boron Removal by Donnan Dialysis: Doehlert Experimental Design. Membranes, 11(10), 731. https://doi.org/10.3390/membranes11100731