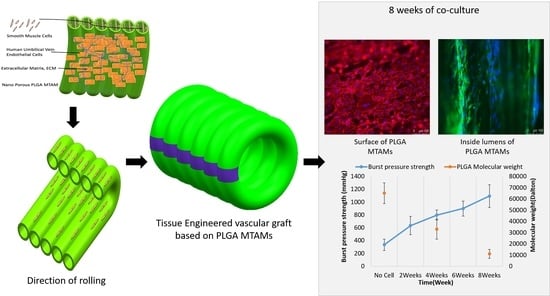
Tissue-Engineered Vascular Graft with Co-Culture of Smooth Muscle Cells and Human Endothelial Vein Cells on an Electrospun Poly(lactic-co-glycolic acid) Microtube Array Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Co-Axial Electrospun PLGA MTAMs
2.2. Microstructure Analysis of Electrospun PLGA MTAMs
2.3. Mechanical Properties of Electrospun PLGA MTAMs
2.4. Plasma Treatment (Acetic Acid; AA)
2.5. Contact Angle Measurement
2.6. Liquid–Liquid Porometry
2.7. SMCs, HUVECs (Cell Culture), and Haptoglobin α-1
2.8. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
2.9. Assembly of TEVGs
2.10. Immunohistochemistry Staining
2.11. Gel Permeation Chromatography (GPC)
2.12. Burst Test of TEVGs
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The Tissue-Engineered Vascular Graft—Past, Present, and Future. Tissue Eng. Part B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef] [PubMed]
- Abdulhannan, P.; Russell, D.; Homer-Vanniasinkam, S. Peripheral arterial disease: A literature review. Br. Med. Bull. 2012, 104, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Dahl, S.L.M.; Kypson, A.P.; Lawson, J.H.; Blum, J.L.; Strader, J.T.; Li, Y.; Manson, R.J.; Tente, W.E.; Dibernardo, L.; Hensley, M.T.; et al. Readily Available Tissue-Engineered Vascular Grafts. Sci. Transl. Med. 2011, 3, 68ra9. [Google Scholar] [CrossRef] [PubMed]
- Lamm, P.; Juchem, G.; Milz, S.; Schuffenhauer, M.; Reichart, B. Autologous Endothelialized Vein Allograft. Circulation 2001, 104, I-108. [Google Scholar] [CrossRef]
- Chard, R.B.; Johnson, D.C.; Nunn, G.R.; Cartmill, T.B. Aorta-coronary bypass grafting with polytetrafluoroethylene conduits. Early and late outcome in eight patients. J. Thorac. Cardiovasc. Surg. 1987, 94, 132–134. [Google Scholar] [CrossRef]
- Hehrlein, F.W.; Schlepper, M.; Loskot, F.; Scheld, H.H.; Walter, P.; Mulch, J. The use of expanded polytetrafluoroethylene (PTFE) grafts for myocardial revascularization. J. Cardiovasc. Surg. 1984, 25, 549–553. [Google Scholar]
- Burkel, W.E.; Vinter, D.W.; Ford, J.W.; Kahn, R.H.; Graham, L.M.; Stanley, J.C. Sequential studies of healing in endothelial seeded vascular prostheses: Histologic and ultrastructure characteristics of graft incorporation. J. Surg. Res. 1981, 30, 305–324. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Salacinski, H.; Hamilton, G.; Seifalian, A. The Mechanical Properties of Infrainguinal Vascular Bypass Grafts: Their Role in Influencing Patency. Eur. J. Vasc. Endovasc. Surg. 2006, 31, 627–636. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, A.; Cheng, K.-S.; Salacinski, H.; Hamilton, G.; Seifalian, A. Improving the patency of vascular bypass grafts: The role of suture materials and surgical techniques on reducing anastomotic compliance mismatch. Eur. J. Vasc. Endovasc. Surg. 2003, 25, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Lemson, M.; Tordoir, J.; Daemen, M.; Kitslaar, P. Intimal hyperplasia in vascular grafts. Eur. J. Vasc. Endovasc. Surg. 2000, 19, 336–350. [Google Scholar] [CrossRef] [Green Version]
- Bussen, S.V. Pathophysiology of Vein Graft Failure: A Review. Radiology 1996, 200, 442. [Google Scholar] [CrossRef]
- Ballyk, P.D.; Walsh, C.; Butany, J.; Ojha, M. Compliance mismatch may promote graft–artery intimal hyperplasia by altering suture-line stresses. J. Biomech. 1997, 31, 229–237. [Google Scholar] [CrossRef]
- Greenwald, S.; Berry, C.L. Improving vascular grafts: The importance of mechanical and haemodynamic properties. J. Pathol. 2000, 190, 292–299. [Google Scholar] [CrossRef]
- Huynh, T.; Abraham, G.; Murray, J.; Brockbank, K.; Hagen, P.-O.; Sullivan, S. Remodeling of an acellular collagen graft into a physiologically responsive neovessel. Nat. Biotechnol. 1999, 17, 1083–1086. [Google Scholar] [CrossRef]
- L’heureux, N.; Pâquet, S.; Labbé, R.; Germain, L.; Auger, F.A. A completely biological tissue-engineered human blood vessel. FASEB J. 1998, 12, 47–56. [Google Scholar]
- Mitchell, S.L.; Niklason, L.E. Requirements for growing tissue-engineered vascular grafts. Cardiovasc. Pathol. 2003, 12, 59–64. [Google Scholar] [CrossRef]
- L’heureux, N.; Dusserre, N.; Marini, A.; Garrido, S.; De La Fuente, L.; McAllister, T. Technology insight: The evolution of tis-sue-engineered vascular grafts--from research to clinical practice. Nat. Rev. Cardiol. 2007, 4, 389. [Google Scholar] [CrossRef] [PubMed]
- Ip, J.H.; Fuster, V.; Badimon, L.; Badimon, J.; Taubman, M.B.; Chesebro, J.H. Syndromes of accelerated atherosclerosis: Role of vascular injury and smooth muscle cell proliferation. J. Am. Coll. Cardiol. 1990, 15, 1667–1687. [Google Scholar] [CrossRef] [Green Version]
- Davies, M.G.; Hagen, P.-O. Pathophysiology of vein graft failure: A review. Eur. J. Vasc. Endovasc. Surg. 1995, 9, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Scharn, D.; Daamen, W.F.; van Kuppevelt, T.; van der Vliet, J. Biological Mechanisms Influencing Prosthetic Bypass Graft Patency: Possible Targets for Modern Graft Design. Eur. J. Vasc. Endovasc. Surg. 2012, 43, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haruguchi, H.; Teraoka, S. Intimal hyperplasia and hemodynamic factors in arterial bypass and arteriovenous grafts: A review. J. Artif. Organs 2003, 6, 227–235. [Google Scholar] [CrossRef]
- Hibino, N.; McGillicuddy, E.; Matsumura, G.; Ichihara, Y.; Naito, Y.; Breuer, C.; Shinoka, T. Late-term results of tissue-engineered vascular grafts in humans. J. Thorac. Cardiovasc. Surg. 2010, 139, 431–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin’Oka, T.; Matsumura, G.; Hibino, N.; Naito, Y.; Watanabe, M.; Konuma, T.; Sakamoto, T.; Nagatsu, M.; Kurosawa, H. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J. Thorac. Cardiovasc. Surg. 2005, 129, 1330–1338. [Google Scholar] [CrossRef] [Green Version]
- Niklason, L.E.; Gao, J.; Abbott, W.M.; Hirschi, K.K.; Houser, S.; Marini, R.; Langer, R. Functional Arteries Grown In Vitro. Science 1999, 284, 489–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoerstrup, S.P.; Mrcs, I.C.; Lachat, M.; Schoen, F.J.; Jenni, R.; Leschka, S.; Neuenschwander, S.; Schmidt, D.; Mol, A.; Günter, C.; et al. Functional Growth in Tissue-Engineered Living, Vascular Grafts. Circulation 2006, 114, I-159–I-166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Allen, R.; Wang, Y. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat. Med. 2012, 18, 1148–1153. [Google Scholar] [CrossRef] [Green Version]
- Yokota, T.; Ichikawa, H.; Matsumiya, G.; Kuratani, T.; Sakaguchi, T.; Iwai, S.; Shirakawa, Y.; Torikai, K.; Saito, A.; Uchimura, E.; et al. In situ tissue regeneration using a novel tis-sue-engineered, small-caliber vascular graft without cell seeding. J. Thorac. Cardiovasc. Surg. 2008, 136, 900–907. [Google Scholar] [CrossRef] [Green Version]
- McAllister, T.N.; Maruszewski, M.; Garrido, S.A.; Wystrychowski, W.; Dusserre, N.; Marini, A.; Zagalski, K.; Fiorillo, A.; Avila, H.; Manglano, X.; et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: A multicentre cohort study. Lancet 2009, 373, 1440–1446. [Google Scholar] [CrossRef]
- Kelm, J.M.; Lorber, V.; Snedeker, J.; Schmidt, D.; Broggini-Tenzer, A.; Weisstanner, M.; Odermatt, B.; Driessen-Mol, A.A.; Zund, G.G.; Hoerstrup, S.P. A novel concept for scaffold-free vessel tissue engineering: Self-assembly of microtissue building blocks. J. Biotechnol. 2010, 148, 46–55. [Google Scholar] [CrossRef]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef] [Green Version]
- Marga, F.; Jakab, K.; Khatiwala, C.; Shepherd, B.; Dorfman, S.; Hubbard, B.; Colbert, S.; Gabor, F. Toward engineering functional organ modules by additive manufacturing. Biofabrication 2012, 4, 022001. [Google Scholar] [CrossRef]
- Shah, P.J.; Bui, K.; Blackmore, S.; Hare, D.L.; Seevanayagam, S.; Gordon, I.; Fuller, J.; Buxton, B.F. Has the in situ right internal thoracic artery been overlooked? An angiographic study of the radial artery, internal thoracic arteries and saphenous vein graft patencies in symptomatic patients? Eur. J. Cardio-Thoracic Surg. 2005, 27, 870–875. [Google Scholar] [CrossRef]
- Lovett, M.; Eng, G.; Kluge, J.A.; Cannizzaro, C.; Vunjak-Novakovic, G.; Kaplan, D.L. Tubular silk scaffolds for small diameter vascular grafts. Organogenesis 2010, 6, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neufurth, M.; Wang, X.; Tolba, E.; Dorweiler, B.; Schröder, H.C.; Link, T.; Diehl-Seifert, B.; Müller, W.E.G. Modular Small Diameter Vascular Grafts with Bioactive Functionalities. PLoS ONE 2015, 10, e0133632. [Google Scholar] [CrossRef] [Green Version]
- Linderman, S.; Araya, J.; Pathan, S.; Nelson, D.; Phaneuf, M.; Contreras, M. A small diameter bioactive prosthetic vascular graft with activated protein C (546.9). FASEB J. 2014, 28, 546.9. [Google Scholar] [CrossRef]
- Enomoto, S.; Sumi, M.; Kajimoto, K.; Nakazawa, Y.; Takahashi, R.; Takabayashi, C.; Asakura, T.; Sata, M. Long-term patency of small-diameter vascular graft made from fibroin, a silk-based biodegradable material. J. Vasc. Surg. 2010, 51, 155–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.-C.; Shu, Y.-C.; Yang, J.-C.; Shie, H.-S.; Lee, S.-Y.; Chen, C.-C. Nano-porous Poly-L-lactic Acid Microtube Array Membranes. Curr. Nanosci. 2014, 10, 227–234. [Google Scholar] [CrossRef]
- Hung, W.-C.; Lin, L.-H.; Tsen, W.-C.; Shie, H.-S.; Chiu, H.-L.; Yang, T.C.-K.; Chen, C.-C. Permeation of biological compounds through porous poly (l-lactic acid) (PLLA) microtube array membranes (MTAMs). Eur. Polym. J. 2015, 67, 166–173. [Google Scholar] [CrossRef]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2013, 10, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Karimi, A.; Navidbakhsh, M.; Shojaei, A.; Faghihi, S. Measurement of the uniaxial mechanical properties of healthy and athero-sclerotic human coronary arteries. Mater. Sci. Eng. C 2013, 33, 2550–2554. [Google Scholar] [CrossRef]
- Yang, J.C.; Lee, S.Y.; Tseng, W.C.; Shu, Y.C.; Lu, J.C.; Shie, H.S.; Chen, C.C. Formation of highly aligned, single-layered, hollow fibrous assemblies and the fabrication of large pieces of PLLA membranes. Macromol. Mater. Eng. 2012, 297, 115–122. [Google Scholar] [CrossRef]
- Latimer, C.A.; Nelson, M.; Moore, C.M.; Martin, K.E. Effect of collagen and elastin content on the burst pressure of human blood vessel seals formed with a bipolar tissue sealing system. J. Surg. Res. 2014, 186, 73–80. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
- Cid, M.C.; Grant, D.S.; Hoffman, G.S.; Auerbach, R.; Fauci, A.S.; Kleinman, H.K. Identification of haptoglobin as an angiogenic factor in sera from patients with systemic vasculitis. J. Clin. Investig. 1993, 91, 977–985. [Google Scholar] [CrossRef] [Green Version]
- Chew, C.H.; Lee, C.-W.; Huang, W.-T.; Cheng, L.-W.; Chen, A.; Cheng, T.-M.; Liu, Y.-L.; Chen, C.-C. Microtube Array Membrane (MTAM)-Based En-capsulated Cell Therapy for Cancer Treatment. Membranes 2020, 10, 80. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Huang, W.-T.; Chew, C.H.; Lai, J.-K.; Tu, S.-H.; Wei, P.-L.; Lee, K.-Y.; Lai, G.-M.; Chen, C.-C. Electrospun Polylactic Acid (PLLA) Microtube Array Membrane (MTAM)—An Advanced Substrate for Anticancer Drug Screening. Materials 2019, 12, 569. [Google Scholar] [CrossRef] [Green Version]
- Tseng, V.C.-H.; Chew, C.H.; Huang, W.-T.; Wang, Y.-K.; Chen, K.-S.; Chou, S.-Y.; Chen, C.-C. An Effective Cell Coculture Platform Based on the Electrospun Microtube Array Membrane for Nerve Regeneration. Cells Tissues Organs 2017, 204, 179–190. [Google Scholar] [CrossRef]
- Walmsley, J.G.; Campling, M.R.; Chertkow, H.M. Interrelationships among wall structure, smooth muscle orientation, and contraction in human major cerebral arteries. Stroke 1983, 14, 781–790. [Google Scholar] [CrossRef] [Green Version]
- Thakar, R.G.; Cheng, Q.; Patel, S.; Chu, J.; Nasir, M.; Liepmann, D.; Komvopoulos, K.; Li, S. Cell-Shape Regulation of Smooth Muscle Cell Proliferation. Biophys. J. 2009, 96, 3423–3432. [Google Scholar] [CrossRef] [Green Version]
- Tian, B.; Ding, X.; Song, Y.; Chen, W.; Liang, J.; Yang, L.; Fan, Y.; Li, S.; Zhou, Y. Matrix stiffness regulates SMC functions via TGF-β signaling pathway. Biomaterials 2019, 221, 119407. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Varkey, M.; Jorgensen, A.; Ju, J.H.; Jin, Q.; Park, J.H.; Fu, Y.; Zhang, G.; Ke, D.; Zhao, W.; et al. Bioprinting small diameter blood vessel constructs with an endothelial and smooth muscle cell bilayer in a single step. Biofabrication 2020, 12, 045012. [Google Scholar] [CrossRef]
- Lim, S.H.; Cho, S.W.; Park, J.C.; Jeon, O.; Lim, J.M.; Kim, S.S.; Kim, B.-S. Tissue-engineered blood vessels with endothelial nitric oxide synthase activity. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 85, 537–546. [Google Scholar] [CrossRef]
- Brennan, M.P.; Dardik, A.; Hibino, N.; Roh, J.D.; Nelson, G.N.; Papademitris, X.; Shinoka, T.; Breuer, C.K. Tissue-engineered Vascular Grafts Demonstrate Evidence of Growth and Development When Implanted in a Juvenile Animal Model. Ann. Surg. 2008, 248, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Lavender, M.D.; Pang, Z.; Wallace, C.S.; Niklason, L.E.; Truskey, G.A. A system for the direct co-culture of endothelium on smooth muscle cells. Biomaterials 2005, 26, 4642–4653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, J.; Huang, Y.; Agarwal, S.; Lannutti, J. Improved Cellular Infiltration in Electrospun Fiber via Engineered Porosity. Tissue Eng. 2007, 13, 2249–2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, B.; Gee, A.O.; Metter, R.B.; Nathan, A.S.; Marklein, R.A.; Burdick, J.A.; Mauck, R.L. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials 2008, 29, 2348–2358. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.I.; Kim, S.Y.; Cho, S.K.; Chong, M.S.; Kim, K.S.; Kim, H.; Lee, S.B.; Lee, Y.M. Tissue-engineered vascular grafts composed of marine collagen and PLGA fibers using pulsatile perfusion bioreactors. Biomaterials 2007, 28, 1115–1122. [Google Scholar] [CrossRef]
- Soffer, L.; Wang, X.; Zhang, X.; Kluge, J.; Dorfmann, L.; Kaplan, D.L.; Leisk, G. Silk-based electrospun tubular scaffolds for tis-sue-engineered vascular grafts. J. Biomater. Sci. Polym. Ed. 2008, 19, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Shin’Oka, T.; Imai, Y.; Ikada, Y. Transplantation of a Tissue-Engineered Pulmonary Artery. N. Engl. J. Med. 2001, 344, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Zamani, F.; Amani-Tehran, M.; Latifi, M.; Shokrgozar, M.A. The influence of surface nanoroughness of electrospun PLGA nano-fibrous scaffold on nerve cell adhesion and proliferation. J. Mater. Sci. Mater. Med. 2013, 24, 1551–1560. [Google Scholar] [CrossRef]
- Lampin, M.; Legris, C.; Degrange, M.; Sigot-Luizard, M.F. Correlation between substratum roughness and wettability, cell adhesion, and cell migration. J. Biomed. Mater. Res. 1997, 36, 99–108. [Google Scholar] [CrossRef]
- Martínez, E.; Engel, E.; Planell, J.; Samitier, J. Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann. Anat. Anat. Anz. 2009, 191, 126–135. [Google Scholar] [CrossRef]
- Ghosh, S.; Spagnoli, G.C.; Martin, I.; Ploegert, S.; Demougin, P.; Heberer, M.; Reschner, A. Three-dimensional culture of melanoma cells profoundly affects gene expression profile: A high density oligonucleotide array study. J. Cell. Physiol. 2005, 204, 522–531. [Google Scholar] [CrossRef]
- Ma, Z.; He, W.; Yong, T.; Ramakrishna, S. Grafting of Gelatin on Electrospun Poly(caprolactone) Nanofibers to Improve Endothelial Cell Spreading and Proliferation and to Control Cell Orientation. Tissue Eng. 2005, 11, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Hajiali, H.; Shahgasempour, S.; Naimi-Jamal, M.R.; Peirovi, H. Electrospun PGA/gelatin nanofibrous scaffolds and their potential application in vascular tissue engineering. Int. J. Nanomed. 2011, 6, 2133–2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Kleijn, D.P.V.; Smeets, M.B.; Kemmeren, P.P.C.W.; Lim, S.K.; Van Middelaar, B.J.; Velema, E.; Schoneveld, A.; Pasterkamp, G.; Borst, C. Acute-phase protein haptoglobin is a cell migration factor involved in arterial restructuring. FASEB J. 2002, 16, 1123–1125. [Google Scholar] [CrossRef]
- Akhtar, R.; Sherratt, M.J.; Cruickshank, J.K.; Derby, B. Characterizing the elastic properties of tissues. Mater. Today 2011, 14, 96–105. [Google Scholar] [CrossRef]
- Murikipudi, S.; Methe, H.; Edelman, E.R. The effect of substrate modulus on the growth and function of matrix-embedded en-dothelial cells. Biomaterials 2013, 34, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Pellegata, A.F.; Asnaghi, M.A.; Stefani, I.; Maestroni, A.; Maestroni, S.; Dominioni, T.; Zonta, S.; Zerbini, G.; Mantero, S. Detergent-Enzymatic Decellularization of Swine Blood Vessels: Insight on Mechanical Properties for Vascular Tissue Engineering. BioMed. Res. Int. 2013, 2013, 918753. [Google Scholar] [CrossRef] [PubMed]
- Konig, G.; McAllister, T.N.; Dusserre, N.; Garrido, S.A.; Iyican, C.; Marini, A.; Fiorillo, A.; Avila, H.; Wystrychowski, W.; Zagalski, K.; et al. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials 2009, 30, 1542–1550. [Google Scholar] [CrossRef] [Green Version]
- Gui, L.; Muto, A.; Chan, S.A.; Breuer, C.; Niklason, L.E. Development of Decellularized Human Umbilical Arteries as Small-Diameter Vascular Grafts. Tissue Eng. Part A 2009, 15, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhou, Q.; Duan, P.; Guo, P.; Li, D.; Xu, Y.; Li, S.; Luo, F.; Zhang, Z. Successful Development of Small Diameter Tissue-Engineering Vascular Vessels by Our Novel Integrally Designed Pulsatile Perfusion-Based Bioreactor. PLoS ONE 2012, 7, e42569. [Google Scholar] [CrossRef] [PubMed] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chew, C.H.; Sheu, B.-L.; Chen, A.; Huang, W.-T.; Cheng, T.-M.; Shih, C.-M.; Chang, A.; Chen, C.-C. Tissue-Engineered Vascular Graft with Co-Culture of Smooth Muscle Cells and Human Endothelial Vein Cells on an Electrospun Poly(lactic-co-glycolic acid) Microtube Array Membrane. Membranes 2021, 11, 732. https://doi.org/10.3390/membranes11100732
Chew CH, Sheu B-L, Chen A, Huang W-T, Cheng T-M, Shih C-M, Chang A, Chen C-C. Tissue-Engineered Vascular Graft with Co-Culture of Smooth Muscle Cells and Human Endothelial Vein Cells on an Electrospun Poly(lactic-co-glycolic acid) Microtube Array Membrane. Membranes. 2021; 11(10):732. https://doi.org/10.3390/membranes11100732
Chicago/Turabian StyleChew, Chee Ho, Bo-Long Sheu, Amanda Chen, Wan-Ting Huang, Tsai-Mu Cheng, Chun-Ming Shih, Austin Chang, and Chien-Chung Chen. 2021. "Tissue-Engineered Vascular Graft with Co-Culture of Smooth Muscle Cells and Human Endothelial Vein Cells on an Electrospun Poly(lactic-co-glycolic acid) Microtube Array Membrane" Membranes 11, no. 10: 732. https://doi.org/10.3390/membranes11100732
APA StyleChew, C. H., Sheu, B. -L., Chen, A., Huang, W. -T., Cheng, T. -M., Shih, C. -M., Chang, A., & Chen, C. -C. (2021). Tissue-Engineered Vascular Graft with Co-Culture of Smooth Muscle Cells and Human Endothelial Vein Cells on an Electrospun Poly(lactic-co-glycolic acid) Microtube Array Membrane. Membranes, 11(10), 732. https://doi.org/10.3390/membranes11100732