Waste Reutilization in Polymeric Membrane Fabrication: A New Direction in Membranes for Separation
Abstract
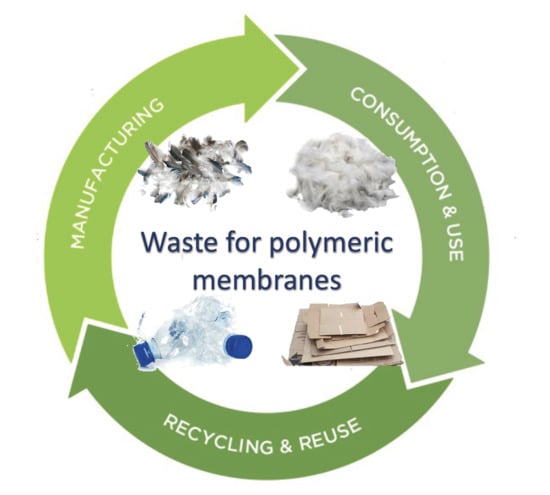
:1. Introduction
2. Potential Waste Sources for Polymeric Membrane Fabrication
2.1. Keratin
2.2. Cellulose
2.3. Plastics and Rubber
3. Development and Performances of Waste-derived Polymeric Membranes for Liquid Separation
3.1. Keratin
3.2. Cellulose and Derivatives
3.3. Polymers and Plastics
4. Challenges and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abu-Bakar, H.; Williams, L.; Hallett, S.H. A review of household water demand management and consumption measurement. J. Clean. Prod. 2021, 292, 125872. [Google Scholar] [CrossRef]
- Muhammad, B. Energy consumption, CO2 emissions and economic growth in developed, emerging and Middle East and North Africa countries. Energy 2019, 179, 232–245. [Google Scholar] [CrossRef]
- Mathai, M.V.; Isenhour, C.; Stevis, D.; Vergragt, P.; Bengtsson, M.; Lorek, S.; Mortensen, L.F.; Coscieme, L.; Scott, D.; Waheed, A.; et al. The Political Economy of (Un)Sustainable Production and Consumption: A Multidisciplinary Synthesis for Research and Action. Resour. Conserv. Recycl. 2021, 167, 105265. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S.; Shetti, N.P.; Kamali, M.; Walvekar, P.; Aminabhavi, T.M. Waste-to-energy nexus: A sustainable development. Environ. Pollut. 2020, 267, 115501. [Google Scholar] [CrossRef] [PubMed]
- Rautela, R.; Arya, S.; Vishwakarma, S.; Lee, J.; Kim, K.H.; Kumar, S. E-waste management and its effects on the environment and human health. Sci. Total Environ. 2021, 773, 145623. [Google Scholar] [CrossRef] [PubMed]
- Akan, O.D.; Udofia, G.E.; Okeke, E.S.; Mgbechidinma, C.L.; Okoye, C.O.; Zoclanclounon, Y.A.B.; Atakpa, E.O.; Adebanjo, O.O. Plastic waste: Status, degradation and microbial management options for Africa. J. Environ. Manag. 2021, 292, 112758. [Google Scholar] [CrossRef] [PubMed]
- Alshehrei, F.; Ameen, F. Vermicomposting: A management tool to mitigate solid waste. Saudi J. Biol. Sci. 2021, 28, 3284–3293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qin, Q.; Li, G.; Tseng, C.H. Sustainable municipal waste management strategies through life cycle assessment method: A review. J. Environ. Manag. 2021, 287, 112238. [Google Scholar] [CrossRef]
- Sharma, H.B.; Vanapalli, K.R.; Samal, B.; Cheela, V.R.S.; Dubey, B.K.; Bhattacharya, J. Circular economy approach in solid waste management system to achieve UN-SDGs: Solutions for post-COVID recovery. Sci. Total Environ. 2021, 800, 149605. [Google Scholar] [CrossRef]
- Luttenberger, L.R. Waste management challenges in transition to circular economy—Case of Croatia. J. Clean. Prod. 2020, 256, 120495. [Google Scholar] [CrossRef]
- Agyabeng-Mensah, Y.; Tang, L.; Afum, E.; Baah, C.; Dacosta, E. Organisational identity and circular economy: Are inter and intra organisational learning, lean management and zero waste practices worth pursuing? Sustain. Prod. Consum. 2021, 28, 648–662. [Google Scholar] [CrossRef]
- Jõgi, K.; Bhat, R. Valorization of food processing wastes and by-products for bioplastic production. Sustain. Chem. Pharm. 2020, 18, 100326. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2017, 434, 60–80. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Ng, B.C.; Abdullah, M.S. Recent progresses of forward osmosis membranes formulation and design for wastewater treatment. Water 2019, 11, 2043. [Google Scholar] [CrossRef] [Green Version]
- Goswami, K.P.; Pugazhenthi, G. Credibility of polymeric and ceramic membrane filtration in the removal of bacteria and virus from water: A review. J. Environ. Manag. 2020, 268, 110583. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.P.; Culfaz-Emecen, P.Z.; Ramon, G.Z.; Visser, T.; Koops, G.H.; Jin, W.; Ulbricht, M. Thinking the future of membranes: Perspectives for advanced and new membrane materials and manufacturing processes. J. Membr. Sci. 2020, 598, 117761. [Google Scholar] [CrossRef]
- Kugarajah, V.; Ojha, A.K.; Ranjan, S.; Dasgupta, N.; Ganesapillai, M.; Dharmalingam, S.; Elmoll, A.; Hosseini, S.A.; Muthulakshmi, L.; Vijayakumar, S.; et al. Future applications of electrospun nanofibers in pressure driven water treatment: A brief review and research update. J. Environ. Chem. Eng. 2021, 9, 105107. [Google Scholar] [CrossRef]
- Sarbatly, R.; Sariau, J.; Alam, M.F.I. Advances in nanofiber membrane. Mater. Today Proc. 2021, 46, 2118–2121. [Google Scholar] [CrossRef]
- Bandehali, S.; Sanaeepur, H.; Ebadi Amooghin, A.; Shirazian, S.; Ramakrishna, S. Biodegradable polymers for membrane separation. Sep. Purif. Technol. 2021, 269, 118731. [Google Scholar] [CrossRef]
- Fredi, G.; Dorigato, A. Recycling of bioplastic waste: A review. Adv. Ind. Eng. Polym. Res. 2021, 4, 159–177. [Google Scholar]
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Sivamani, S.; Sivakumar, N.; Maran, J.P.; Hosseini-Bandegharaei, A. Ecofriendly biopolymers and composites: Preparation and their applications in water-treatment. Biotechnol. Adv. 2021, 52, 107815. [Google Scholar] [CrossRef]
- Patel, S.H.; Xanthos, M. Environmental Issues in Polymer Processing: A Review on Volatile Emissions and Material/Energy Recovery Options; Springer: Berlin/Heidelberg, Germany, 2001; Volume 20. [Google Scholar]
- Mansoori, S.; Davarnejad, R.; Matsuura, T.; Ismail, A.F. Membranes based on non-synthetic (natural) polymers for wastewater treatment. Polym. Test. 2020, 84, 106381. [Google Scholar] [CrossRef]
- Debnath, B.; Haldar, D.; Purkait, M.K. A critical review on the techniques used for the synthesis and applications of crystalline cellulose derived from agricultural wastes and forest residues. Carbohydr. Polym. 2021, 273, 118537. [Google Scholar] [CrossRef] [PubMed]
- Bushra, R.; Mohamad, S.; Alias, Y.; Jin, Y.; Ahmad, M. Current approaches and methodologies to explore the perceptive adsorption mechanism of dyes on low-cost agricultural waste: A review. Microporous Mesoporous Mater. 2021, 319, 111040. [Google Scholar] [CrossRef]
- Freitas, L.C.; Barbosa, J.R.; da Costa, A.L.C.; Bezerra, F.W.F.; Pinto, R.H.H.; de Carvalho Junior, R.N. From waste to sustainable industry: How can agro-industrial wastes help in the development of new products? Resour. Conserv. Recycl. 2021, 169, 105466. [Google Scholar] [CrossRef]
- Kwikima, M.M.; Mateso, S.; Chebude, Y. Potentials of Agricultural wastes as the ultimate alternative adsorbent for Cadmium removal from wastewater. A review. Sci. Afr. 2021, 13, e00934. [Google Scholar]
- Lewoyehu, M. Comprehensive Review on Synthesis and Application of Activated Carbon from Agricultural Residues for the Remediation of Venomous Pollutants in Wastewater. J. Anal. Appl. Pyrolysis 2021, 159, 105279. [Google Scholar] [CrossRef]
- Solangi, N.H.; Kumar, J.; Mazari, S.A.; Ahmed, S.; Fatima, N.; Mubarak, N.M. Development of fruit waste derived bio-adsorbents for wastewater treatment: A review. J. Hazard. Mater. 2021, 416, 125848. [Google Scholar] [CrossRef]
- Kadhom, M.; Albayati, N.; Alalwan, H.; Al-Furaiji, M. Removal of dyes by agricultural waste. Sustain. Chem. Pharm. 2020, 16, 100259. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, F.; Wu, Y.R. Emerging technologies for conversion of sustainable algal biomass into value-added products: A state-of-the-art review. Sci. Total Environ. 2021, 784, 147024. [Google Scholar] [CrossRef]
- Yan, G.; Chen, B.; Zeng, X.; Sun, Y.; Tang, X.; Lin, L. Recent advances on sustainable cellulosic materials for pharmaceutical carrier applications. Carbohydr. Polym. 2020, 244, 116492. [Google Scholar] [CrossRef]
- Abdulyekeen, K.A.; Umar, A.A.; Patah, M.F.A.; Daud, W.M.A.W. Torrefaction of biomass: Production of enhanced solid biofuel from municipal solid waste and other types of biomass. Renew. Sustain. Energy Rev. 2021, 150, 111436. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Sirohi, R.; Varjani, S.; Hyoun Kim, S.; Wong, J.W.C. Sustainable processing of food waste for production of bio-based products for circular bioeconomy. Bioresour. Technol. 2021, 325, 124684. [Google Scholar] [CrossRef]
- Jannat, N.; Hussien, A.; Abdullah, B.; Cotgrave, A. Application of agro and non-agro waste materials for unfired earth blocks construction: A review. Constr. Build. Mater. 2020, 254, 119346. [Google Scholar] [CrossRef]
- Al-Fakih, A.; Mohammed, B.S.; Liew, M.S.; Nikbakht, E. Incorporation of waste materials in the manufacture of masonry bricks: An update review. J. Build. Eng. 2019, 21, 37–54. [Google Scholar] [CrossRef]
- Li, L.; Zuo, J.; Duan, X.; Wang, S.; Hu, K.; Chang, R. Impacts and mitigation measures of plastic waste: A critical review. Environ. Impact Assess. Rev. 2021, 90, 106642. [Google Scholar] [CrossRef]
- Sharma, B.; Shekhar, S.; Sharma, S.; Jain, P. The paradigm in conversion of plastic waste into value added materials. Clean. Eng. Technol. 2021, 4, 100254. [Google Scholar] [CrossRef]
- Al Rayaan, M.B. Recent advancements of thermochemical conversion of plastic waste to biofuel-A review. Clean. Eng. Technol. 2021, 2, 100062. [Google Scholar] [CrossRef]
- Zulkernain, N.H.; Gani, P.; Chuck Chuan, N.; Uvarajan, T. Utilisation of plastic waste as aggregate in construction materials: A review. Constr. Build. Mater. 2021, 296, 123669. [Google Scholar] [CrossRef]
- Wang, J.; Qian, W.; He, Y.; Xiong, Y.; Song, P.; Wang, R.M. Reutilization of discarded biomass for preparing functional polymer materials. Waste Manag. 2017, 65, 11–21. [Google Scholar] [CrossRef]
- Maiti, A.; Pandey, A. Polymer and Waste Plastic in Membranes. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Donato, R.K.; Mija, A. Keratin associations with synthetic, biosynthetic and natural polymers: An extensive review. Polymers 2020, 12, 32. [Google Scholar] [CrossRef] [Green Version]
- Reddy, C.C.; Khilji, I.A.; Gupta, A.; Bhuyar, P.; Mahmood, S.; AL-Japairai, K.A.S.; Chua, G.K. Valorization of keratin waste biomass and its potential applications. J. Water Process Eng. 2021, 40, 101707. [Google Scholar] [CrossRef]
- Shavandi, A.; Silva, T.H.; Bekhit, A.A.; Bekhit, A.E.D.A. Keratin: Dissolution, extraction and biomedical application. Biomater. Sci. 2017, 5, 1699–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donner, M.W.; Arshad, M.; Ullah, A.; Siddique, T. Unravelled keratin-derived biopolymers as novel biosorbents for the simultaneous removal of multiple trace metals from industrial wastewater. Sci. Total Environ. 2019, 647, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Gaidau, C.; Epure, D.G.; Enascuta, C.E.; Carsote, C.; Sendrea, C.; Proietti, N.; Chen, W.; Gu, H. Wool keratin total solubilisation for recovery and reintegration—An ecological approach. J. Clean. Prod. 2019, 236, 117586. [Google Scholar] [CrossRef]
- Ma, B.; Qiao, X.; Hou, X.; Yang, Y. Pure keratin membrane and fibres from chicken feather. Int. J. Biol. Macromol. 2016, 89, 614–621. [Google Scholar] [CrossRef] [Green Version]
- Holkar, C.R.; Jain, S.S.; Jadhav, A.J.; Pinjari, D.V. Valorization of keratin based waste. Process Saf. Environ. Prot. 2018, 115, 85–98. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Mikula, K.; Witek-Krowiak, A.; Izydorczyk, G.; Kuligowski, K.; Bandrów, P.; Kułażyński, M. Progress in sustainable technologies of leather wastes valorization as solutions for the circular economy. J. Clean. Prod. 2021, 313, 127902. [Google Scholar] [CrossRef]
- Aluigi, A.; Sotgiu, G.; Torreggiani, A.; Guerrini, A.; Orlandi, V.T.; Corticelli, F.; Varchi, G. Methylene Blue Doped Films of Wool Keratin with Antimicrobial Photodynamic Activity. ACS Appl. Mater. Interfaces 2015, 7, 17416–17424. [Google Scholar] [CrossRef]
- Balaji, S.; Kumar, R.; Sripriya, R.; Kakkar, P.; Ramesh, D.V.; Reddy, P.N.K.; Sehgal, P.K. Preparation and comparative characterization of keratin-chitosan and keratin-gelatin composite scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2012, 32, 975–982. [Google Scholar] [CrossRef]
- Park, M.; Shin, H.K.; Kim, B.S.; Kim, M.J.; Kim, I.S.; Park, B.Y.; Kim, H.Y. Effect of discarded keratin-based biocomposite hydrogels on the wound healing process in vivo. Mater. Sci. Eng. C 2015, 55, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.O.S.; Carletto, R.A.; Tonetti, C.; Giachet, F.T.; Varesano, A.; Vineis, C. Wool keratin film plasticized by citric acid for food packaging. Food Packag. Shelf Life 2017, 12, 100–106. [Google Scholar] [CrossRef]
- Feroz, S.; Muhammad, N.; Ranayake, J.; Dias, G. Keratin—Based materials for biomedical applications. Bioact. Mater. 2020, 5, 496–509. [Google Scholar] [CrossRef]
- Lazarus, B.S.; Chadha, C.; Velasco-Hogan, A.; Barbosa, J.D.V.; Jasiuk, I.; Meyers, M.A. Engineering with keratin: A functional material and a source of bioinspiration. iScience 2021, 24, 102798. [Google Scholar] [CrossRef]
- Rajabi, M.; Ali, A.; McConnell, M.; Cabral, J. Keratinous materials: Structures and functions in biomedical applications. Mater. Sci. Eng. C 2020, 110, 110612. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, A.; Chik, S.M.S.T.; Kee, C.G.; Mistry, B.M.; Kim, D.H.; Sharma, G. Characterization of keratin microparticles from feather biomass with potent antioxidant and anticancer activities. Int. J. Biol. Macromol. 2017, 104, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Tran, C.D.; Mututuvari, T.M. Cellulose, Chitosan and Keratin Composite Materials: Facile and Recyclable Synthesis, Conformation and Properties. ACS Sustain. Chem. Eng. 2016, 4, 1850–1861. [Google Scholar] [CrossRef]
- Esparza, Y.; Bandara, N.; Ullah, A.; Wu, J. Hydrogels from feather keratin show higher viscoelastic properties and cell proliferation than those from hair and wool keratins. Mater. Sci. Eng. C 2018, 90, 446–453. [Google Scholar] [CrossRef]
- Ozaki, Y.; Takagi, Y.; Mori, H.; Hara, M. Porous hydrogel of wool keratin prepared by a novel method: An extraction with guanidine/2-mercaptoethanol solution followed by a dialysis. Mater. Sci. Eng. C 2014, 42, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Sun, Y.; Chen, H.; Xu, C.; Hu, Y. An ultrasonic-ionic liquid process for the efficient acid catalyzed hydrolysis of feather keratin. Chin. J. Chem. Eng. 2019, 27, 660–667. [Google Scholar] [CrossRef]
- Schindl, A.; Hagen, M.L.; Muzammal, S.; Gunasekera, H.A.D.; Croft, A.K. Proteins in ionic liquids: Reactions, applications, and futures. Front. Chem. 2019, 7, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idris, A.; Vijayaraghavan, R.; Rana, U.A.; Patti, A.F.; MacFarlane, D.R. Dissolution and regeneration of wool keratin in ionic liquids. Green Chem. 2014, 16, 2857–2864. [Google Scholar] [CrossRef]
- Jiang, Z.; Yuan, J.; Wang, P.; Fan, X.; Xu, J.; Wang, Q.; Zhang, L. Dissolution and regeneration of wool keratin in the deep eutectic solvent of choline chloride-urea. Int. J. Biol. Macromol. 2018, 119, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Nuutinen, E.M.; Willberg-Keyriläinen, P.; Virtanen, T.; Mija, A.; Kuutti, L.; Lantto, R.; Jääskeläinen, A.S. Green process to regenerate keratin from feathers with an aqueous deep eutectic solvent. RSC Adv. 2019, 9, 19720–19728. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Li, S.; Zhang, S. Ionic liquids as novel solvents for the dissolution and blending of wool keratin fibres. Green Chem. 2005, 7, 606–608. [Google Scholar] [CrossRef]
- Brown, E.M.; Pandya, K.; Taylor, M.M.; Liu, C.-K. Comparison of Methods for Extraction of Keratin from Waste Wool. Agric. Sci. 2016, 7, 670–679. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.; Zhang, D.; Wei, Y.; Zhao, Z.; Ma, X.; Zhao, X.; Wang, S.; Yang, W. Preparation of Ag doped keratin/PA6 nanofiber membrane with enhanced air filtration and antimicrobial properties. Polymers 2019, 11, 1511. [Google Scholar] [CrossRef] [Green Version]
- Sinkiewicz, I.; Śliwińska, A.; Staroszczyk, H.; Kołodziejska, I. Alternative Methods of Preparation of Soluble Keratin from Chicken Feathers. Waste Biomass Valorization 2017, 8, 1043–1048. [Google Scholar] [CrossRef]
- Fagbemi, O.D.; Sithole, B.; Tesfaye, T. Optimization of keratin protein extraction from waste chicken feathers using hybrid pre-treatment techniques. Sustain. Chem. Pharm. 2020, 17, 100267. [Google Scholar] [CrossRef]
- Saha, S.; Zubair, M.; Khosa, M.A.; Song, S.; Ullah, A. Keratin and Chitosan Biosorbents for Wastewater Treatment: A Review. J. Polym. Environ. 2019, 27, 1389–1403. [Google Scholar] [CrossRef]
- Khosa, M.A.; Wu, J.; Ullah, A. Chemical modification, characterization, and application of chicken feathers as novel biosorbents. RSC Adv. 2013, 3, 20800–20810. [Google Scholar] [CrossRef] [Green Version]
- Hussain, F.S.; Memon, N.; Khatri, Z.; Memon, S. Solid waste-derived biodegradable keratin sponges for removal of chromium: A circular approach for waste management in leather industry. Environ. Technol. Innov. 2020, 20, 101120. [Google Scholar] [CrossRef]
- Zahara, I.; Arshad, M.; Naeth, M.A.; Siddique, T.; Ullah, A. Feather keratin derived sorbents for the treatment of wastewater produced during energy generation processes. Chemosphere 2021, 273, 128545. [Google Scholar] [CrossRef] [PubMed]
- Aluigi, A.; Rombaldoni, F.; Tonetti, C.; Jannoke, L. Study of Methylene Blue adsorption on keratin nanofibrous membranes. J. Hazard. Mater. 2014, 268, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Aluigi, A.; Varesano, A.; Vineis, C.; del Rio, A. Electrospinning of immiscible systems: The wool keratin/polyamide-6 case study. Mater. Des. 2017, 127, 144–153. [Google Scholar] [CrossRef]
- Ariffin, H.; Hassan, M.A.; Shah, U.K.M.; Abdullah, N.; Ghazali, F.M.; Shirai, Y. Production of bacterial endoglucanase from pretreated oil palm empty fruit bunch by bacillus pumilus EB3. J. Biosci. Bioeng. 2008, 106, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Phromphithak, S.; Onsree, T.; Tippayawong, N. Machine learning prediction of cellulose-rich materials from biomass pretreatment with ionic liquid solvents. Bioresour. Technol. 2021, 323, 124642. [Google Scholar] [CrossRef] [PubMed]
- Baghel, R.S.; Reddy, C.R.K.; Singh, R.P. Seaweed-based cellulose: Applications, and future perspectives. Carbohydr. Polym. 2021, 267, 118241. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.S.; Li, Y.H. Maximizing the yield of nanocrystalline cellulose from cotton pulp fiber. Carbohydr. Polym. 2012, 88, 1184–1188. [Google Scholar] [CrossRef]
- Srasri, K.; Thongroj, M.; Chaijiraaree, P.; Thiangtham, S.; Manuspiya, H.; Pisitsak, P.; Ummartyotin, S. Recovery potential of cellulose fiber from newspaper waste: An approach on magnetic cellulose aerogel for dye adsorption material. Int. J. Biol. Macromol. 2018, 119, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Medronho, B.; Lindman, B. Brief overview on cellulose dissolution/regeneration interactions and mechanisms. Adv. Colloid Interface Sci. 2015, 222, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Yao, Z.; Wang, X.; Crombeen, M.; Sweeney, D.G.; Tam, K.C. Cellulose-based materials in wastewater treatment of petroleum industry. Green Energy Environ. 2020, 5, 37–49. [Google Scholar] [CrossRef]
- Homem, N.C.; Amorim, M.T.P. Synthesis of cellulose acetate using as raw material textile wastes. In Materials Today: Proceedings; Elsevier Ltd: Amsterdam, The Netherlands, 2019; Volume 31, pp. S315–S317. [Google Scholar]
- Eduok, U.; Abdelrasoul, A.; Shoker, A.; Doan, H. Recent developments, current challenges and future perspectives on cellulosic hemodialysis membranes for highly efficient clearance of uremic toxins. Mater. Today Commun. 2021, 27, 102183. [Google Scholar] [CrossRef]
- Silva, M.A.; Belmonte-Reche, E.; de Amorim, M.T.P. Morphology and water flux of produced cellulose acetate membranes reinforced by the design of experiments (DOE). Carbohydr. Polym. 2021, 254, 117407. [Google Scholar] [CrossRef]
- Chevalier, Q.; el Hadri, H.; Petitjean, P.; Bouhnik-Le Coz, M.; Reynaud, S.; Grassl, B.; Gigault, J. Nano-litter from cigarette butts: Environmental implications and urgent consideration. Chemosphere 2018, 194, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Puls, J.; Wilson, S.A.; Hölter, D. Degradation of Cellulose Acetate-Based Materials: A Review. J. Polym. Environ. 2011, 19, 152–165. [Google Scholar] [CrossRef] [Green Version]
- Rebischung, F.; Chabot, L.; Biaudet, H.; Pandard, P. Cigarette butts: A small but hazardous waste, according to European regulation. Waste Manag. 2018, 82, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Livazovic, S.; Li, Z.; Behzad, A.R.; Peinemann, K.V.; Nunes, S.P. Cellulose multilayer membranes manufacture with ionic liquid. J. Membr. Sci. 2015, 490, 282–293. [Google Scholar] [CrossRef] [Green Version]
- Ventura-Cruz, S.; Tecante, A. Nanocellulose and microcrystalline cellulose from agricultural waste: Review on isolation and application as reinforcement in polymeric matrices. Food Hydrocoll. 2021, 118, 106771. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, G.; Zhang, H.; Zhao, C.; Yang, F. Improvement of antifouling performances for modified PVDF ultrafiltration membrane with hydrophilic cellulose nanocrystal. Appl. Surf. Sci. 2018, 440, 1091–1100. [Google Scholar] [CrossRef]
- Iskandar, M.J.; Baharum, A.; Anuar, F.H.; Othaman, R. Palm oil industry in South East Asia and the effluent treatment technology—A review. Environ. Technol. Innov. 2018, 9, 169–185. [Google Scholar] [CrossRef]
- Teh, K.C.; Foo, M.L.; Ooi, C.W.; Leng Chew, I.M. Sustainable and cost-effective approach for the synthesis of lignin-containing cellulose nanocrystals from oil palm empty fruit bunch. Chemosphere 2021, 267, 129277. [Google Scholar] [CrossRef] [PubMed]
- Rasli, S.R.A.M.; Ahmad, I.; Lazim, A.M.; Hamzah, A. Pengekstrakan dan pencirian selulosa daripada bahan buangan pertanian—Pelepah kelapa sawit. Malays. J. Anal. Sci. 2017, 21, 1065–1073. [Google Scholar]
- Shanmugarajah, B.; Chew, I.M.L.; Mubarak, N.M.; Choong, T.S.Y.; Yoo, C.K.; Tan, K.W. Valorization of palm oil agro-waste into cellulose biosorbents for highly effective textile effluent remediation. J. Clean. Prod. 2019, 210, 697–709. [Google Scholar] [CrossRef]
- Foo, M.L.; Ooi, C.W.; Tan, K.W.; Chew, I.M.L. A Step Closer to Sustainable Industrial Production: Tailor the Properties of Nanocrystalline Cellulose from Oil Palm Empty Fruit Bunch. J. Environ. Chem. Eng. 2020, 8, 104058. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Liu, T.; Liu, T.; Zhang, J.; Liu, H. Eco-friendly post-consumer cotton waste recycling for regenerated cellulose fibres. Carbohydr. Polym. 2019, 206, 141–148. [Google Scholar] [CrossRef]
- Neelamegam, A.; Al-Battashi, H.; Al-Bahry, S.; Nallusamy, S. Biorefinery production of poly-3-hydroxybutyrate using waste office paper hydrolysate as feedstock for microbial fermentation. J. Biotechnol. 2018, 265, 25–30. [Google Scholar] [CrossRef]
- Xia, G.; Wan, J.; Zhang, J.; Zhang, X.; Xu, L.; Wu, J.; He, J.; Zhang, J. Cellulose-based films prepared directly from waste newspapers via an ionic liquid. Carbohydr. Polym. 2016, 151, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Zhang, S.; Li, F.; Yang, Y.; Du, M. Recent advances in cellulose-based membranes for their sensing applications. Cellulose 2020, 27, 9157–9179. [Google Scholar] [CrossRef]
- Budtova, T.; Navard, P. Cellulose in NaOH–water based solvents: A review. Cellulose 2016, 23, 5–55. [Google Scholar] [CrossRef] [Green Version]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem.—Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, Y.; Xu, H.; Yang, Q.; Xiong, C.; Kuga, S.; Matsumoto, Y. Facile dissolution of wood pulp in aqueous NaOH/urea solution by ball milling pretreatment. Ind. Crop. Prod. 2018, 118, 48–52. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; You, T.; Wang, K.; Xu, F. Effects of polymorphs on dissolution of cellulose in NaOH/urea aqueous solution. Carbohydr. Polym. 2015, 125, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Zhang, C.; Kang, H.; Huang, Y.; Liu, R. Extensional rheology of cellulose/NaOH/urea/H2O solutions. Cellulose 2016, 23, 2877–2885. [Google Scholar] [CrossRef]
- Liu, G.; Li, W.; Chen, L.; Zhang, X.; Niu, D.; Chen, Y.; Yuan, S.; Bei, Y.; Zhu, Q. Molecular dynamics studies on the aggregating behaviors of cellulose molecules in NaOH/urea aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124663. [Google Scholar] [CrossRef]
- Xiong, B.; Zhao, P.; Hu, K.; Zhang, L.; Cheng, G. Dissolution of cellulose in aqueous NaOH/urea solution: Role of urea. Cellulose 2014, 21, 1183–1192. [Google Scholar] [CrossRef]
- Liu, G.; Sun, H.; Liu, G.; Zhang, H.; Yuan, S.; Zhu, Q. A molecular dynamics study of cellulose inclusion complexes in NaOH/urea aqueous solution. Carbohydr. Polym. 2018, 185, 12–18. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic liquids and cellulose: Dissolution, chemical modification and preparation of new cellulosic materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef]
- Anokhina, T.S.; Pleshivtseva, T.S.; Ignatenko, V.Y.; Antonov, S.V.; Volkov, A.V. Fabrication of composite nanofiltration membranes from cellulose solutions in an [Emim]OAc–DMSO mixture. Pet. Chem. 2017, 57, 477–482. [Google Scholar] [CrossRef]
- Gericke, M.; Fardim, P.; Heinze, T. Ionic liquids—Promising but challenging solvents for homogeneous derivatization of cellulose. Molecules 2012, 17, 7458–7502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uto, T.; Yamamoto, K.; Kadokawa, J.I. Cellulose Crystal Dissolution in Imidazolium-Based Ionic Liquids: A Theoretical Study. J. Phys. Chem. B 2018, 122, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Glińska, K.; Gitalt, J.; Torrens, E.; Plechkova, N.; Bengoa, C. Extraction of cellulose from corn stover using designed ionic liquids with improved reusing capabilities. Process Saf. Environ. Prot. 2021, 147, 181–191. [Google Scholar] [CrossRef]
- Kasavan, S.; Yusoff, S.; Rahmat Fakri, M.F.; Siron, R. Plastic pollution in water ecosystems: A bibliometric analysis from 2000 to 2020. J. Clean. Prod. 2021, 313, 127946. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Li, W.C.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total. Environ. 2016, 566–567, 333–349. [Google Scholar] [CrossRef]
- Kubowicz, S.; Booth, A.M. Biodegradability of Plastics: Challenges and Misconceptions. Environ. Sci. Technol. 2017, 51, 12058–12060. [Google Scholar] [CrossRef]
- Lin, C.H.; Gung, C.H.; Wu, J.Y.; Suen, S.Y. Cationic dye adsorption using porous composite membrane prepared from plastic and plant wastes. J. Taiwan Inst. Chem. Eng. 2015, 51, 119–126. [Google Scholar] [CrossRef]
- Li, G.; Wang, J.; Hou, D.; Bai, Y.; Liu, H. Fabrication and performance of PET mesh enhanced cellulose acetate membranes for forward osmosis. J. Environ. Sci. 2015, 45, 7–17. [Google Scholar] [CrossRef]
- Zander, N.E.; Gillan, M.; Sweetser, D. Recycled PET nanofibers for water filtration applications. Materials 2016, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Ghazanfari, D.; Bastani, D.; Mousavi, S.A. Preparation and characterization of poly (vinyl chloride) (PVC) based membrane for wastewater treatment. J. Water Process Eng. 2017, 16, 98–107. [Google Scholar] [CrossRef]
- El-Gendi, A.; Abdallah, H.; Amin, A.; Amin, S.K. Investigation of polyvinylchloride and cellulose acetate blend membranes for desalination. J. Mol. Struct. 2017, 1146, 14–22. [Google Scholar] [CrossRef]
- Demirel, E.; Zhang, B.; Papakyriakou, M.; Xia, S.; Chen, Y. Fe2O3 nanocomposite PVC membrane with enhanced properties and separation performance. J. Membr. Sci. 2017, 529, 170–184. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Ivars, J.; Wang-Xu, X.; Iborra-Clar, M.I. Application of post-consumer recycled high-impact polystyrene in the preparation of phase-inversion membranes for low-pressure membrane processes. Sep. Purif. Technol. 2017, 175, 340–351. [Google Scholar] [CrossRef]
- Wu, H.; Li, T.; Liu, B.; Chen, C.; Wang, S.; Crittenden, J.C. Blended PVC/PVC-g-PEGMA ultrafiltration membranes with enhanced performance and antifouling properties. Appl. Surf. Sci. 2018, 455, 987–996. [Google Scholar] [CrossRef]
- Rajesh, S.; Murthy, Z.V.P. Ultrafiltration membranes from waste polyethylene terephthalate and additives: Synthesis and characterization. Quim. Nova 2014, 37, 653–657. [Google Scholar] [CrossRef]
- Vaysizadeh, A.; Zinatizadeh, A.A.; Zinadini, S. Fouling mitigation and enhanced dye rejection in UF and NF membranes via layer-by-layer (LBL) assembly and altering PVP percentage as pore former. Environ. Technol. Innov. 2021, 23, 101698. [Google Scholar] [CrossRef]
- Mamah, S.C.; Goh, P.S.; Ismail, A.F.; Suzaimi, N.D.; Ahmad, N.A.; Lee, W.J. Flux enhancement in reverse osmosis membranes induced by synergistic effect of incorporated palygorskite/chitin hybrid nanomaterial. J. Environ. Chem. Eng. 2021, 9, 105432. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Waste rubber recycling: A review on the evolution and properties of thermoplastic elastomers. Materials 2020, 13, 782. [Google Scholar] [CrossRef] [Green Version]
- Bockstal, L.; Berchem, T.; Schmetz, Q.; Richel, A. Devulcanisation and reclaiming of tires and rubber by physical and chemical processes: A review. J. Clean. Prod. 2019, 236, 117574. [Google Scholar] [CrossRef]
- Ma, H.; Shen, J.; Cao, J.; Wang, D.; Yue, B.; Mao, Z.; Wu, W.; Zhang, H. Fabrication of wool keratin/polyethylene oxide nano-membrane from wool fabric waste. J. Clean. Prod. 2017, 161, 357–361. [Google Scholar] [CrossRef]
- Ding, J.; Chen, M.; Chen, W.; He, M.; Zhou, X.; Yin, G. Vapor-assisted crosslinking of a FK/PVA/PEO nanofiber membrane. Polymers 2018, 10, 747. [Google Scholar] [CrossRef] [Green Version]
- Zhong, X.; Li, R.; Wang, Z.; Wang, W.; Yu, D. Eco-fabrication of antibacterial nanofibrous membrane with high moisture permeability from wasted wool fabrics. Waste Manag. 2020, 102, 404–411. [Google Scholar] [CrossRef]
- David, P.S.; Karunanithi, A.; Fathima, N.N. Improved filtration for dye removal using keratin–polyamide blend nanofibrous membranes. Environ. Sci. Pollut. Res. 2020, 27, 45629–45638. [Google Scholar] [CrossRef]
- Karunanidhi, A.; David, P.S.; Fathima, N.N. Electrospun Keratin-Polysulfone Blend Membranes for Treatment of Tannery Effluents. Water Air Soil Pollut. 2020, 231, 1–11. [Google Scholar] [CrossRef]
- Teow, Y.H.; Amirudin, S.N.; Ho, K.C. Sustainable approach to the synthesis of cellulose membrane from oil palm empty fruit bunch for dye wastewater treatment. J. Water Process Eng. 2020, 34, 101182. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Ismail, A.F.; Abd Mutalib, M.; Mohamad, A.B.; Zain, M.F.; Awang, N.A.; Mohd Hir, Z.A. Physicochemical characterization of cellulose nanocrystal and nanoporous self-assembled CNC membrane derived from Ceiba pentandra. Carbohydr. Polym. 2017, 157, 1892–1902. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, A.; Anugwom, I.; Blot, H.; Sanchez Conde, A.; Manttari, M.; Kallioinen, M. Re-use of waste cotton textile as an ultrafiltration membrane. J. Environ. Chem. Eng. 2021, 9, 105705. [Google Scholar] [CrossRef]
- Vignesh, N.; Suriyaraj, S.P.; Selvakumar, R.; Chandraraj, K. Facile Fabrication and Characterization of Zn Loaded Cellulose Membrane from Cotton Microdust Waste and its Antibacterial Properties—A Waste to Value Approach. J. Polym. Environ. 2021, 29, 1651–1662. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.; Jaafar, J.; Ismail, A.F.; Mutalib, M.A.; Sani, N.A.A.; Asri, S.E.A.M.; Ong, C.S. Physicochemical characteristic of regenerated cellulose/N-doped TiO2 nanocomposite membrane fabricated from recycled newspaper with photocatalytic activity under UV and visible light irradiation. Chem. Eng. J. 2016, 284, 202–215. [Google Scholar] [CrossRef]
- Campano, C.; Miranda, R.; Merayo, N.; Negro, C.; Blanco, A. Direct production of cellulose nanocrystals from old newspapers and recycled newsprint. Carbohydr. Polym. 2017, 173, 489–496. [Google Scholar] [CrossRef]
- Rodrigues Filho, G.; Monteiro, D.S.; da Silva Meireles, C.; de Assunção, R.M.N.; Cerqueira, D.A.; Barud, H.S.; Ribeiro, S.J.L.; Messadeq, Y. Synthesis and characterization of cellulose acetate produced from recycled newspaper. Carbohydr. Polym. 2008, 73, 74–82. [Google Scholar] [CrossRef]
- Ünlü, C.H. Carboxymethylcellulose from recycled newspaper in aqueous medium. Carbohydr. Polym. 2013, 97, 159–164. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Ismail, A.F.; Abd Mutalib, M.; Jamil, S.M. Feasibility of recycled newspaper as cellulose source for regenerated cellulose membrane fabrication. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Liu, W.; Cui, M.; Shen, Y.; Zhu, G.; Luo, L.; Li, M.; Li, J. Waste cigarette filter as nanofibrous membranes for on-demand immiscible oil/water mixtures and emulsions separation. J. Colloid Interface Sci. 2019, 549, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Doyan, A.; Leong, C.L.; Bilad, M.R.; Kurnia, K.A.; Susilawati, S.; Prayogi, S.; Narkkun, T.; Faungnawakij, K. Cigarette butt waste as material for phase inverted membrane fabrication used for oil/water emulsion separation. Polymers 2021, 13, 1907. [Google Scholar] [CrossRef] [PubMed]
- Aji, M.M.; Narendren, S.; Purkait, M.K.; Katiyar, V. Utilization of waste polyvinyl chloride (PVC) for ultrafiltration membrane fabrication and its characterization. J. Environ. Chem. Eng. 2020, 8, 103650. [Google Scholar] [CrossRef]
- Mishra, G.; Mukhopadhyay, M. Enhanced antifouling performance of halloysite nanotubes (HNTs) blended poly(vinyl chloride) (PVC/HNTs) ultrafiltration membranes: For water treatment. J. Ind. Eng. Chem. 2018, 63, 366–379. [Google Scholar] [CrossRef]
- Aji, M.M.; Narendren, S.; Purkait, M.K.; Katiyar, V. Biopolymer (gum arabic) incorporation in waste polyvinylchloride membrane for the enhancement of hydrophilicity and natural organic matter removal in water. J. Water Process Eng. 2020, 38. [Google Scholar] [CrossRef]
- Wang, S.Y.; Fang, L.F.; Cheng, L.; Jeon, S.; Kato, N.; Matsuyama, H. Novel ultrafiltration membranes with excellent antifouling properties and chlorine resistance using a poly(vinyl chloride)-based copolymer. J. Membr. Sci. 2018, 549, 101–110. [Google Scholar] [CrossRef]
- Fang, L.-F.; Jeon, S.; Kakihana, Y.; Kakehi, J.-i.; Zhu, B.-K.; Matsuyama, H.; Zhao, S. Improved antifouling properties of polyvinyl chloride blend membranes by novel phosphate based-zwitterionic polymer additive. J. Membr. Sci. 2017, 528, 326–335. [Google Scholar] [CrossRef]
- Adamczak, M.; Kamińska, G.; Bohdziewicz, J. Application of waste polymers as basic material for ultrafiltration membranes preparation. Water 2020, 12, 179. [Google Scholar] [CrossRef] [Green Version]
- Kusumocahyo, S.P.; Ambani, S.K.; Kusumadewi, S.; Sutanto, H.; Widiputri, D.I.; Kartawiria, I.S. Utilization of used polyethylene terephthalate (PET) bottles for the development of ultrafiltration membrane. J. Environ. Chem. Eng. 2020, 8, 104381. [Google Scholar] [CrossRef]
- Mulyati, S.; Armando, M.A.; Mawardi, H.; Fahrina, A.; Malahayati, N.; Muchtar, S. Fabrication of hydrophilic and strong pet-based membrane from wasted plastic bottle. Rasayan J. Chem. 2018, 11, 1609–1617. [Google Scholar] [CrossRef]
- Kiani, S.; Mousavi, S.M.; Bidaki, A. Preparation of polyethylene terephthalate/xanthan nanofiltration membranes using recycled bottles for removal of diltiazem from aqueous solution. J. Clean. Prod. 2021, 314, 128082. [Google Scholar] [CrossRef]
- Dong, L.X.; Huang, X.C.; Wang, Z.; Yang, Z.; Wang, X.M.; Tang, C.Y. A thin-film nanocomposite nanofiltration membrane prepared on a support with in situ embedded zeolite nanoparticles. Sep. Purif. Technol. 2016, 166, 230–239. [Google Scholar] [CrossRef]
- Xu, G.R.; An, X.C.; Das, R.; Xu, K.; Xing, Y.L.; Hu, Y.X. Application of electrospun nanofibrous amphiphobic membrane using low-cost poly (ethylene terephthalate) for robust membrane distillation. J. Water Process Eng. 2020, 36, 101351. [Google Scholar] [CrossRef]
- Doan, H.N.; Phong Vo, P.; Hayashi, K.; Kinashi, K.; Sakai, W.; Tsutsumi, N. Recycled PET as a PDMS-Functionalized electrospun fibrous membrane for oil-water separation. J. Environ. Chem. Eng. 2020, 8, 103921. [Google Scholar] [CrossRef]
- Pulido, B.A.; Habboub, O.S.; Aristizabal, S.L.; Szekely, G.; Nunes, S.P. Recycled Poly(ethylene terephthalate) for High Temperature Solvent Resistant Membranes. ACS Appl. Polym. Mater. 2019, 1, 2379–2387. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.T.; Kao, F.Y.; Chen, S.H.; Wey, M.Y.; Tseng, H.H. A facile approach from waste to resource: Reclaimed rubber-derived membrane for dye removal. J. Taiwan Inst. Chem. Eng. 2020, 112, 286–295. [Google Scholar] [CrossRef]
- Gan, L.; Qiu, F.; Yue, X.; Chen, Y.; Xu, J.; Zhang, T. Aramid nanofiber aerogel membrane extract from waste plastic for efficient separation of surfactant-stabilized oil-in-water emulsions. J. Environ. Chem. Eng. 2021, 9, 106137. [Google Scholar] [CrossRef]
- Nakasone, K.; Kobayashi, T. Cytocompatible cellulose hydrogels containing trace lignin. Mater. Sci. Eng. C 2016, 64, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Hamad, K.; Kaseem, M.; Deri, F. Recycling of waste from polymer materials: An overview of the recent works. Polym. Degrad. Stab. 2013, 98, 2801–2812. [Google Scholar] [CrossRef]
- Xie, W.; Li, T.; Tiraferri, A.; Drioli, E.; Figoli, A.; Crittenden, J.C.; Liu, B. Toward the Next Generation of Sustainable Membranes from Green Chemistry Principles. ACS Sustain. Chem. Eng. 2021, 9, 50–75. [Google Scholar] [CrossRef]
- Yadav, P.; Ismail, N.; Essalhi, M.; Tysklind, M.; Athanassiadis, D.; Tavajohi, N. Assessment of the environmental impact of polymeric membrane production. J. Membr. Sci. 2021, 622, 118987. [Google Scholar] [CrossRef]
- Lee, W.J.; Goh, P.S.; Lau, W.J.; Ismail, A.F.; Hilal, N. Green approaches for sustainable development of liquid separation membrane. Membranes 2021, 11, 235. [Google Scholar] [CrossRef]
- Gupta, S.K.; Mao, Y. A review on molten salt synthesis of metal oxide nanomaterials: Status, opportunity, and challenge. Prog. Mater. Sci. 2021, 117, 100734. [Google Scholar] [CrossRef]









| Materials | Solvent | Water Source | Membrane | Removal/Separation | Performance Indicator | Refs. |
|---|---|---|---|---|---|---|
| Keratin | IL | Dyed wool fabric | NF nanofibre membrane | - | Bacteria inhibition rate: E. coli: 89.2% S. aureus: 60.7 | [137] |
| Keratin | Formic acid | Goat hair | Polyamide/keratin electro spun membrane | Tannery dye removal | Rejection: 100% | [138] |
| Cellulose | NMP | OPEFB | UF | Dye removal | MB removal: 34.9% | [140] |
| Cellulose | - | Kapok fibre | Self-assembled CNC membrane | Dye removal | MB removal: 85% | [141] |
| Cellulose | DMSO | Non-dye cotton bed sheet | UF | - | PEG 35 kD rejection: ~90% | [142] |
| Cellulose | NaOH/urea | Recycled newspaper | UF | - | PWP: 0.35 L m−2 h−1 bar−1 | [148] |
| Cellulose | NaOH/urea | Recycled newspaper | Photocatalytic membrane, TiO2 nanorod additive | Phenol removal | Phenol degradation: 96.6% (UV) 78.8% (Visible light) PWF: 4.12 L m−2 h−1 | [144] |
| Cellulose | DMF | Waste cigarette filter | Electrospun stainless steel supported membrane | Oil/water emulsion separation | Separation efficiency: >99% Flux: > 100 L m−2 h−1 | [149] |
| Cellulose | DMF | Waste cigarette filter | UF | Oil/water emulsion separation | Separation efficiency: >94% P: > 180 L m−2 h−1 bar−1 | [150] |
| PVC | NMP | Campus post-consumer disposal | UF, GA additive | Humic acid removal | HA rejection: 96% Flux: 98 L m−2 h−1 | [153] |
| PET | Phenol | Post-consumer bottle | UF, PEG4000 additive | BSA removal | BSA rejection: 90% | [158] |
| HIPS | DMF | Recycled | UF | Humic acid removal | RT= 49% HA rejection: 96% | [128] |
| PS | DMF | Packaging filling | UF | Micropollutant-containing river wastewater treatment | Phenolic compound rejection: ~40% Colour rejection: ~70% | [157] |
| PET | TFA | Post-consumer bottle | NF, XA additive | Diltiazem-containing solution | Diltiazem rejection: | [156] |
| PET | TFA | Post-consumer soft drink bottle | Electrospun NF, hot pressed and fluorinated | Desalination | Permeation: 11−23 L m−2 h−1 Salt rejection: >99.9% | [161] |
| PET | TFA and dichloromethane (DCM) | Post-consumer bottle | Electrospun NF, PDMS coating | Oil/water separation | Flux: 20,000 L m−2 h−1 Separation efficiency: >98% | [162] |
| Rubber | Toluene | Recycled, unvulcanised tyre | NF | MB removal | PWP: 1596.1 L m−2 h−1 bar−1 Permeability: 10.6 L m−2 h−1 bar−1 MB rejection: 93% | [163] |
| Aramid fibre | KOH/DMSO | Kevlar | Aerogel membrane | Oil/water emulsion separation | Separation efficiency: >98% Flux: > 1940 L m−2 h−1 | [164] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, P.S.; Othman, M.H.D.; Matsuura, T. Waste Reutilization in Polymeric Membrane Fabrication: A New Direction in Membranes for Separation. Membranes 2021, 11, 782. https://doi.org/10.3390/membranes11100782
Goh PS, Othman MHD, Matsuura T. Waste Reutilization in Polymeric Membrane Fabrication: A New Direction in Membranes for Separation. Membranes. 2021; 11(10):782. https://doi.org/10.3390/membranes11100782
Chicago/Turabian StyleGoh, Pei Sean, Mohd Hafiz Dzarfan Othman, and Takeshi Matsuura. 2021. "Waste Reutilization in Polymeric Membrane Fabrication: A New Direction in Membranes for Separation" Membranes 11, no. 10: 782. https://doi.org/10.3390/membranes11100782
APA StyleGoh, P. S., Othman, M. H. D., & Matsuura, T. (2021). Waste Reutilization in Polymeric Membrane Fabrication: A New Direction in Membranes for Separation. Membranes, 11(10), 782. https://doi.org/10.3390/membranes11100782