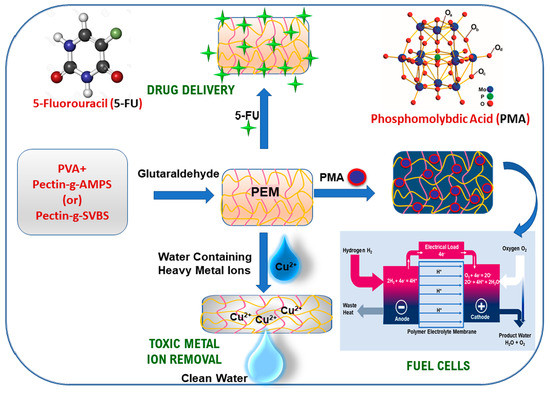
Fabrication of Polyelectrolyte Membranes of Pectin Graft-Copolymers with PVA and Their Composites with Phosphomolybdic Acid for Drug Delivery, Toxic Metal Ion Removal, and Fuel Cell Applications
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of PEMs
2.3. Swelling Studies
2.4. 5-Fluorouracil Encapsulation Efficiency and In Vitro Drug Release Studies
2.5. Copper Ion Removal
2.6. Ion Exchange Capacity, Proton Conductivity, and Methanol Permeability Studies
2.7. Characterization
3. Results
3.1. Synthesis of Graft-Copolymers
3.2. FTIR Studies
3.3. XRD Studies
3.4. SEM and EDAX Studies
3.5. Water Uptake Measurements
3.6. 5-Fluorouracil Release Studies
3.7. Copper Metal Ion Adsorption Studies
3.8. Proton Conductivity and Methanol Permeability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, X.; Zhang, Z.; Yu, D.; Chen, X.; Cheng, R.; Min, S.; Wang, J.; Xiao, Q.; Wang, J. Overview of membrane technology applications for industrial wastewater treatment in China to increase water supply. Resour. Conserv. Recycl. 2015, 105, 1–10. [Google Scholar] [CrossRef]
- Xiao, K.; Liang, S.; Wang, X.; Chen, C.; Huang, X. Current state and challenges of full-scale membrane bioreactor applications: A critical review. Bioresour. Technol. 2019, 271, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Wandera, D.; Wickramasinghe, S.R.; Husson, S.M. Stimuli-responsive membranes. J. Membr. Sci. 2010, 357, 6–35. [Google Scholar] [CrossRef]
- Tahara, Y.; Nakashi, K.; Ji, K.; Ikeda, A.; Toko, K. Development of a portable taste sensor with a lipid/polymer membrane. Sensors 2013, 13, 1076–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiam, H.S.; Daud, W.R.W.; Kamarudin, S.K.; Mohammad, A.B.; Kadhum, A.A.H.; Loh, K.S.; Majlan, E.H. Overview on nanostructured membrane in fuel cell applications. Int. J. Hydrog. Energy 2011, 36, 3187–3205. [Google Scholar] [CrossRef]
- Rao, K.K.; Naidu, B.V.K.; Subha, M.C.S.; Sairam, M.; Mallikarjuna, N.N.; Aminabahvi, T.M. Novel carbohydrate polymeric blend membranes in pervaporation dehydration of acetic acid. Carbohydr. Polym. 2006, 66, 345–351. [Google Scholar]
- Fadhil, S. Performance of Nanofiltration Membranes on Water DemineralizationAssessment and Comparative Study. Indian J. Adv. Chem. Sci. 2018, 6, 178–181. [Google Scholar]
- Almeida Júnior, J.H.S.; Bertuol, D.A.; Meneguzzi, A.; Ferreira, C.A.; Amado, F.D.R. Castor oil and commercial thermoplastic polyurethane membranes modified with polyaniline: A comparative study. Mater. Res. 2013, 16, 860–866. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.R.S.; Rao, K.M.; Rao, K.S.V.K.; Shchipunov, Y.; Ha, C.S. Synthesis of alginate based silver nanocomposite hydrogels for biomedical applications. Macromol. Res. 2014, 22, 832–842. [Google Scholar] [CrossRef]
- Gao, L.; Li, C.; Huang, W.; Mei, S.; Lin, H.; Ou, Q.; Zhang, Y.; Guo, J.; Zhang, F.; Xu, S.; et al. MXene/polymer membranes: Synthesis, properties, and emerging applications. Chem. Mater. 2020, 32, 1703–1747. [Google Scholar] [CrossRef]
- Silva, C.L.; Pereira, J.C.; Ramalho, A.; Pais, A.A.C.C.; Sousa, J.J.S. Films based on chitosan polyelectrolyte complexes for skin drug delivery: Development and characterization. J. Membr. Sci. 2008, 320, 268–279. [Google Scholar] [CrossRef] [Green Version]
- Detzel, C.J.; Larkin, B.S.A.L.; Rajagopalan, P. Polyelectrolyte multilayers in tissue engineering. Tissue Eng. Part B Rev. 2011, 17, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Rao, K.S.V.K.; Subha, M.C.S.; Sairam, M.; Mallikarjuna, N.N.; Aminabhavi, T.M. Blend membranes of chitosan and poly (vinyl alcohol) in pervaporation dehydration of isopropanol and tetrahydrofuran. J. Appl. Polym. Sci. 2007, 103, 1918–1926. [Google Scholar] [CrossRef]
- Zhao, Q.; An, Q.F.; Ji, Y.; Qian, J.; Gao, C. Polyelectrolyte complex membranes for pervaporation, nanofiltration and fuel cell applications. J. Membr. Sci. 2011, 379, 19–45. [Google Scholar] [CrossRef]
- Lee, C.-H.; Terbish, N.; Holder, S.L.; Popuri, S.R.; Nalluri, L.P. A study on development of alternative biopolymers based proton exchange membrane for microbial fuel cells and effect of blending ratio and ionic crosslinking on bioenergy generation and COD removal. J. Polym. Res. 2019, 26, 285. [Google Scholar] [CrossRef]
- Vani, T.J.S.; Reddy, N.S.; Reddy, P.R.; Rao, K.S.V.K.; Ramkumar, J.; Reddy, A.V.R. Synthesis, characterization, and metal uptake capacity of a new polyaniline and poly (acrylic acid) grafted sodium alginate/gelatin adsorbent. Desalin. Water Treat. 2014, 52, 526–535. [Google Scholar] [CrossRef]
- Eldin, M.S.M.; Hashem, A.E.; Tamer, T.M.; Omer, A.M.; Yossuf, M.E.; Sabet, M.M. Development of cross linked chi-tosan/alginate polyelectrolyte proton exchanger membranes for fuel cell applications. Int. J. Electrochem. Sci. 2017, 12, 3840–3858. [Google Scholar] [CrossRef]
- Dittenber, D.B.; GangaRao, H.V.S. Critical review of recent publications on use of natural composites in infrastructure. Compo. Part A Appl. Sci. Manuf. 2012, 43, 1419–1429. [Google Scholar] [CrossRef]
- Gouveia, T.I.A.; Biernacki, K.; Castro, M.C.R.; Gonçalves, M.P.; Souza, H.K.S. A new approach to develop biodegradable films based on thermoplastic pectin. Food Hydrocoll. 2019, 97, 105175. [Google Scholar] [CrossRef]
- Eswaramma, S.; Reddy, N.S.; Rao, K.S.V.K. Phosphate crosslinked pectin based dual responsive hydrogel networks and nanocomposites: Development, swelling dynamics and drug release characteristics. Int. J. Biol. Macromol. 2017, 103, 1162–1172. [Google Scholar] [CrossRef]
- Reddy, N.S.; Rao, K.M.; Vani, T.J.S.; Rao, K.S.V.K.; Lee, Y.I. Pectin/poly (acrylamide-co-acrylamidoglycolic acid) pH sensitive semi-IPN hydrogels: Selective removal of Cu2+ and Ni2+, modeling, and kinetic studies. Desalin. Water Treat. 2016, 57, 6503–6514. [Google Scholar] [CrossRef]
- Reddy, P.; Eswaramma, S.; Rao, K.S.V.K.; Lee, Y.I. Dual responsive pectin hydrogels and their silver nanocomposites: Swelling studies, controlled drug delivery and antimicrobial applications. Bull. Korean Chem. Soc. 2014, 35, 2391–2399. [Google Scholar] [CrossRef] [Green Version]
- Van Baelen, D.; Van der Bruggen, B.; Van den Dungen, K.; Degreve, J.; Vandecasteele, C. Pervaporation of water–alcohol mixtures and acetic acid–water mixtures. Chem. Eng. Sci. 2005, 60, 1583–1590. [Google Scholar] [CrossRef]
- Haaz, E.; Toth, A.J. Methanol dehydration with pervaporation: Experiments and modeling. Sep. Purif. Technol. 2018, 205, 121–129. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, Q.G.; Han, G.L.; Zhu, A.M.; Liu, Q.L. Pervaporation of water–ethanol and methanol–MTBE mixtures using poly (vinyl alcohol)/cellulose acetate blended membranes. J. Membr. Sci. 2013, 448, 93–101. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Hanafiah, M.M.; Ashraf, M.A.; Hanafiah, M.M. Sustaining life on earth system through clean air, pure water, and fertile soil. Environ. Sci. Pollut. Res. 2019, 26, 13679–13680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohd Nizam, N.U.; Mohd Hanafiah, M.; Mohd Noor, I.; Abd Karim, H.I. Efficiency of five selected aquatic plants in phy-toremediation of aquaculture wastewater. Appl. Sci. 2020, 10, 2712. [Google Scholar] [CrossRef] [Green Version]
- Al-Raad, A.A.; Hanafiah, M.M.; Naje, A.S.; Ajeel, M.A. Optimized parameters of the electrocoagulation process using a novel reactor with rotating anode for saline water treatment. Environ. Pollut. 2020, 265, 115049. [Google Scholar] [CrossRef]
- Hanafiah, M.M.; Hashim, N.A.; Ahmed, S.T.; Muhammad, A.A. Removal of chromium from aqueous solutions using a palm kernel shell adsorbent. Desalin. Water Treat. 2018, 18, 172–180. [Google Scholar] [CrossRef]
- Manikam, M.K.; Halim, A.A.; Hanafiah, M.M.; Krishnamoorthy, R.R. Removal of ammonia nitrogen, nitrate, phosphorus and COD from sewage wastewater using palm oil boiler ash composite adsorbent. Desalin. Water Treat. 2019, 149, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Lima, A.T.; Ottosen, L.M.; Ribeiro, A.B. Assessing fly ash treatment: Remediation and stabilization of heavy metals. J. Environ. Manag. 2012, 95, S110–S115. [Google Scholar] [CrossRef]
- Reddy, N.S.; Rao, K.S.V.K. Polymeric hydrogels: Recent advances in toxic metal ion removal and anticancer drug delivery applications. Indian J. Adv. Chem. Sci. 2016, 4, 214–234. [Google Scholar]
- Bejan, D.; Bunce, N.J. Acid mine drainage: Electrochemical approaches to prevention and remediation of acidity and toxic metals. J. Appl. Electrochem. 2015, 45, 1239–1254. [Google Scholar] [CrossRef]
- Breeze, P. Power Generation Technologies. Newnes: Burlington, MA, USA, 2019. [Google Scholar]
- Junior, O.H.A.; Maran, A.L.O.; Henao, N.C. A review of the development and applications of thermoelectric microgenerators for energy harvesting. Renew. Sustain. Energy Rev. 2018, 91, 376–393. [Google Scholar] [CrossRef]
- Lam, D.V.; Jo, K.; Kim, C.-H.; Kim, J.-H.; Lee, H.-J.; Lee, S.-M. Activated carbon textile via chemistry of metal extraction for supercapacitors. ACS Nano 2016, 10, 11351–11359. [Google Scholar] [CrossRef]
- Pan, M.; Pan, C.; Li, C.; Zhao, J. A review of membranes in proton exchange membrane fuel cells: Transport phenomena, performance and durability. Renew. Sustain. Energy Rev. 2021, 141, 110771. [Google Scholar]
- Ion-Ebrasu, D.; Pollet, B.G.; Spinu-Zaulet, A.; Soare, A.; Carcadea, E.; Varlam, M.; Caprarescu, S. Graphene modified fluori-nated cation-exchange membranes for proton exchange membrane water electrolysis. Int. J. Hydrogen Energy 2019, 44, 10190–10196. [Google Scholar] [CrossRef]
- Zuo, P.; Li, Y.; Wang, A.; Tan, R.; Liu, Y.; Liang, X.; Sheng, F.; Tang, G.; Ge, L.; Wu, L.; et al. Sulfonated microporous polymer membranes with fast and selective ion transport for electrochemical energy conversion and storage. Angew. Chem. Int. Ed. 2020, 59, 9564–9573. [Google Scholar] [CrossRef]
- Prasad, S.S.; Rao, K.M.; Reddy, P.R.S.; Reddy, N.S.; Rao, K.S.V.K.; Subha, M.C.S. Synthesis and characterisation of guar gum-g-poly (acrylamidoglycolic acid) by redox initiator. Indian J. Adv. Chem. Sci. 2012, 1, 28–32. [Google Scholar]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Shaari, N.; Kamarudin, S.K.; Basri, S.; Shyuan, L.K.; Masdar, M.S.; Nordin, D. Enhanced mechanical flexibility and perfor-mance of sodium alginate polymer electrolyte bio-membrane for application in direct methanol fuel cell. J. Appl. Polym. Sci. 2018, 135, 46666. [Google Scholar] [CrossRef]
- Rambabu, G.; Bhat, S.D. Amino acid functionalized graphene oxide based nanocomposite membrane electrolytes for direct methanol fuel cells. J. Membr. Sci. 2018, 551, 1–11. [Google Scholar] [CrossRef]
- Kourasi, M.; Wills, R.G.A.; Shah, A.A.; Walsh, F.C. Heteropolyacids for fuel cell applications. Electrochim. Acta 2014, 127, 454–466. [Google Scholar] [CrossRef]
- Bary, E.M.A.; Soliman, Y.A.; Fekri, A.; Harmal, A.N. Aging of novel membranes made of PVA and cellulose nanocrystals extracted from Egyptian rice husk manufactured by compression moulding process. Int. J. Environ. Stud. 2018, 75, 750–762. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, L.; Han, L. Polymer-nanoinorganic particles composite membranes: A brief overview. Front. Chem. Eng. China 2009, 3, 318–329. [Google Scholar] [CrossRef]
- Guo, R.; Hu, C.; Pan, F.; Wu, H.; Jiang, Z. PVA–GPTMS/TEOS hybrid pervaporation membrane for dehydration of ethylene glycol aqueous solution. J. Membr. Sci. 2006, 281, 454–462. [Google Scholar] [CrossRef]
- Chaiwarit, T.; Rachtanapun, P.; Kantrong, N.; Jantrawut, P. Preparation of clindamycin hydrochloride loaded de-esterified low-methoxyl mango peel pectin film used as a topical drug delivery system. Polymers 2020, 12, 1006. [Google Scholar] [CrossRef]
- Meng, Y.-J.; Wang, S.-Y.; Guo, Z.-W.; Cheng, M.-M.; Li, J.; Li, D.-Q. Design and preparation of quaternized pec-tin-Montmorillonite hybrid film for sustained drug release. Int. J. Biol. Macromol. 2020, 154, 413–420. [Google Scholar] [CrossRef]
- Wang, S.; Meng, Y.; Li, J.; Liu, J.; Liu, Z.; Li, D. A novel and simple oral colon-specific drug delivery system based on the pectin/modified nano-carbon sphere nanocomposite gel films. Int. J. Biol. Macromol. 2020, 157, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Ngah, W.S.W.; Endud, C.S.; Mayanar, R. Removal of copper (II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React. Funct. Polym. 2002, 50, 181–190. [Google Scholar] [CrossRef]
- Martins, J.G.; Facchi, D.P.; Berton, S.B.R.; Nunes, C.S.; Matsushita, M.; Bonafé, E.G.; Popat, K.C.; Almeida, V.C.; Kipper, M.J.; Martins, A.F. Removal of Cu (II) from aqueous solutions imparted by a pectin-based film: Cytocompatibility, antimicrobial, kinetic, and equilibrium studies. Int. J. Biol. Macromol. 2020, 152, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Misono, M. Heterogeneous catalysis by heteropoly compounds of molybdenum and tungsten. Catal. Rev. Sci. Eng. 1987, 29, 269–321. [Google Scholar] [CrossRef]
- Bielański, A.; Micek-Ilnicka, A. Kinetics and mechanism of gas phase MTBE and ETBE formation on Keggin and Wells–Dawson heteropolyacids as catalysts. Inorg. Chim. Acta 2010, 363, 4158–4162. [Google Scholar] [CrossRef]








| PEM Code | %ESR | IEC | Proton Conductivity (σ)∗10−3 (S/cm) | Methanol Permeability 10−5 (cm−2/s) |
|---|---|---|---|---|
| PPCAM | 140 | 0.50 | 7.81 | 5.17 |
| PPCAM-PMA | 82 | 0.59 | 17.01 | 2.77 |
| PPCSB | 350 | 0.41 | 13.77 | 6.83 |
| PPCSB-PMA | 72 | 0.67 | 18.6 | 5.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijitha, R.; Reddy, N.S.; Nagaraja, K.; Vani, T.J.S.; Hanafiah, M.M.; Venkateswarlu, K.; Lakkaboyana, S.K.; Rao, K.S.V.K.; Rao, K.M. Fabrication of Polyelectrolyte Membranes of Pectin Graft-Copolymers with PVA and Their Composites with Phosphomolybdic Acid for Drug Delivery, Toxic Metal Ion Removal, and Fuel Cell Applications. Membranes 2021, 11, 792. https://doi.org/10.3390/membranes11100792
Vijitha R, Reddy NS, Nagaraja K, Vani TJS, Hanafiah MM, Venkateswarlu K, Lakkaboyana SK, Rao KSVK, Rao KM. Fabrication of Polyelectrolyte Membranes of Pectin Graft-Copolymers with PVA and Their Composites with Phosphomolybdic Acid for Drug Delivery, Toxic Metal Ion Removal, and Fuel Cell Applications. Membranes. 2021; 11(10):792. https://doi.org/10.3390/membranes11100792
Chicago/Turabian StyleVijitha, Raagala, Nagella Sivagangi Reddy, Kasula Nagaraja, Tiruchuru J. Sudha Vani, Marlia M. Hanafiah, Katta Venkateswarlu, Sivarama Krishna Lakkaboyana, Kummari S. V. Krishna Rao, and Kummara Madhususdana Rao. 2021. "Fabrication of Polyelectrolyte Membranes of Pectin Graft-Copolymers with PVA and Their Composites with Phosphomolybdic Acid for Drug Delivery, Toxic Metal Ion Removal, and Fuel Cell Applications" Membranes 11, no. 10: 792. https://doi.org/10.3390/membranes11100792
APA StyleVijitha, R., Reddy, N. S., Nagaraja, K., Vani, T. J. S., Hanafiah, M. M., Venkateswarlu, K., Lakkaboyana, S. K., Rao, K. S. V. K., & Rao, K. M. (2021). Fabrication of Polyelectrolyte Membranes of Pectin Graft-Copolymers with PVA and Their Composites with Phosphomolybdic Acid for Drug Delivery, Toxic Metal Ion Removal, and Fuel Cell Applications. Membranes, 11(10), 792. https://doi.org/10.3390/membranes11100792