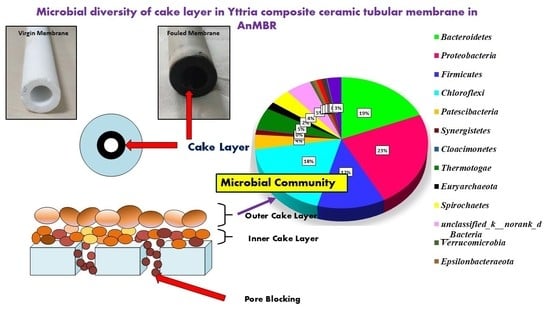
New Insights into the Microbial Diversity of Cake Layer in Yttria Composite Ceramic Tubular Membrane in an Anaerobic Membrane Bioreactor (AnMBR)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of MBR
2.2. Cake Layer Sampling and Membrane Chemical Cleaning
2.3. Microbial Community Analysis
2.4. Excitation-Emission Matrix (EEM) Fluorescence Spectroscopy
2.5. Cake Layer Resistance Calculation
3. Results and Discussions
3.1. AnCMBR Performance and Microbial Community Evolution
3.2. Cake Layer Resistance and Composition
3.3. Identification of Key Bacteria and Archaea in the Cake Layer
3.3.1. Bacteria in Phylum Level in the Cake Layer
3.3.2. Top Archaea in Family Level in Cake Layer
3.3.3. Bacterial Community in Genus Level in the Cake Layer
3.3.4. Top Archaea in Genus Level in Cake Layer
3.4. Comparison of the Bulk Sludge and the Cake Layer
3.4.1. The Bacterial Diversity in Bulk Sludge Versus Cake Layers in Phylum Level
3.4.2. Archaea Community in Bulk Sludge Versus Cake Layer
3.5. The Presence of Bacteria and Archaeal in Cleaning Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, D.W.; Zhang, T.; Tang, C.-Y.Y.; Wu, W.-M.; Wong, C.-Y.; Lee, Y.H.; Yeh, D.H.; Criddle, C.S. Membrane fouling in an anaerobic membrane bioreactor: Differences in relative abundance of bacterial species in the membrane foulant layer and in suspension. J. Membr. Sci. 2010, 364, 331–338. [Google Scholar] [CrossRef]
- Cashman, S.; Ma, X.; Mosley, J.; Garland, J.; Crone, B.; Xue, X. Energy and greenhouse gas life cycle assessment and cost analysis of aerobic and anaerobic membrane bioreactor systems: Influence of scale, population density, climate, and methane recovery. Bioresour. Technol. 2018, 254, 56–66. [Google Scholar] [CrossRef]
- Kong, Z.; Li, L.; Xue, Y.; Yang, M.; Li, Y.-Y. Challenges and prospects for the anaerobic treatment of chemical-industrial organic wastewater: A review. J. Clean. Prod. 2019, 231, 913–927. [Google Scholar] [CrossRef]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-based membranes for water and wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578. [Google Scholar] [CrossRef]
- Sun, H.; Liu, H.; Wang, S.; Cheng, F.; Liu, Y. Ceramic membrane fouling by dissolved organic matter generated during on-line chemical cleaning with ozone in MBR. Water Res. 2018, 146, 328–336. [Google Scholar] [CrossRef]
- Ghyoot, W.R.; Verstraete, W.H. Coupling Membrane Filtration to Anaerobic Primary Sludge Digestion. Environ. Technol. 1997, 18, 569–580. [Google Scholar] [CrossRef]
- Ahmad, R.; Aslam, M.; Park, E.; Chang, S.; Kwon, D.; Kim, J. Submerged low-cost pyrophyllite ceramic membrane filtration combined with GAC as fluidized particles for industrial wastewater treatment. Chemosphere 2018, 206, 784–792. [Google Scholar] [CrossRef]
- Mei, X.; Quek, P.J.; Wang, Z.; Ng, H.Y. Alkali-assisted membrane cleaning for fouling control of anaerobic ceramic membrane bioreactor. Bioresour. Technol. 2017, 240, 25–32. [Google Scholar] [CrossRef]
- Yue, X.; Koh, Y.K.; Ng, H.Y. Treatment of domestic wastewater with an anaerobic ceramic membrane bioreactor (AnCMBR). Water Sci. Technol. 2015, 72, 2301–2307. [Google Scholar] [CrossRef]
- Jeong, Y.; Hermanowicz, S.W.; Park, C. Treatment of food waste recycling wastewater using anaerobic ceramic membrane bioreactor for biogas production in mainstream treatment process of domestic wastewater. Water Res. 2017, 123, 86–95. [Google Scholar] [CrossRef]
- Nilusha, R.T.; Yu, D.; Zhang, J.; Wei, Y. Effects of Solids Retention Time on the Anaerobic Membrane Bioreactor with Yttria-Based Ceramic Membrane Treating Domestic Wastewater at Ambient Temperature. Membranes 2020, 10. [Google Scholar] [CrossRef]
- Nilusha, R.T.; Wang, T.; Wang, H.; Yu, D.; Zhang, J.; Wei, Y. Optimization of In Situ Backwashing Frequency for Stable Operation of Anaerobic Ceramic Membrane Bioreactor. Processes 2020, 8, 545. [Google Scholar] [CrossRef]
- Koyuncu, I.; Sengur-Tasdemir, R.; Ersahin, M.E.; Ozgun, H.; Kose-Mutlu, B.; Turken, T.; Kaya, R.; Yavuzturk-Gul, B. Applications of Ceramic Membrane Bioreactors in Water Treatment. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 141–176. [Google Scholar]
- Gkotsis, P.K.; Zouboulis, A.I. Biomass Characteristics and Their Effect on Membrane Bioreactor Fouling. Molecules 2019, 24. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Robledo, M.; Cid-León, D.M.; Morgan-Sagastume, J.M.; Noyola, A. Biofouling in an anaerobic membrane bioreactor treating municipal sewage. Sep. Purif. Technol. 2011, 81, 49–55. [Google Scholar] [CrossRef]
- Takada, K.; Shiba, T.; Yamaguchi, T.; Akane, Y.; Nakayama, Y.; Soda, S.; Inoue, D.; Ike, M. Cake layer bacterial communities during different biofouling stages in full-scale membrane bioreactors. Bioresour. Technol. 2018, 259, 259–267. [Google Scholar] [CrossRef]
- Wu, Y.; Xia, Y.; Jing, X.; Cai, P.; Igalavithana, A.D.; Tang, C.; Tsang, D.C.W.; Ok, Y.S. Recent advances in mitigating membrane biofouling using carbon-based materials. J. Hazard. Mater. 2020, 382, 120976. [Google Scholar] [CrossRef]
- Wood, T.L.; Guha, R.; Tang, L.; Geitner, M.; Kumar, M.; Wood, T.K. Living biofouling-resistant membranes as a model for the beneficial use of engineered biofilms. Proc. Natl. Acad. Sci. USA 2016, 113, E2802–E2811. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Kim, E.S.; Ahn, Y. Microbial community analysis of bulk sludge/cake layers and biofouling-causing microbial consortia in a full-scale aerobic membrane bioreactor. Bioresour. Technol. 2017, 227, 133–141. [Google Scholar] [CrossRef]
- Aslam, M.; Ahmad, R.; Kim, J. Recent developments in biofouling control in membrane bioreactors for domestic wastewater treatment. Sep. Purif. Technol. 2018, 206, 297–315. [Google Scholar] [CrossRef]
- Baek, Y.; Yu, J.; Kim, S.-H.; Lee, S.; Yoon, J. Effect of surface properties of reverse osmosis membranes on biofouling occurrence under filtration conditions. J. Membr. Sci. 2011, 382, 91–99. [Google Scholar] [CrossRef]
- Moran, A.P. Microbial Glycobiology; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Zeuner, B.; Ovtar, S.; Persson, Å.H.; Foghmoes, S.; Berendt, K.; Ma, N.; Kaiser, A.; Negra, M.D.; Pinelo, M. Surface treatments and functionalization of metal-ceramic membranes for improved enzyme immobilization performance. J. Chem. Technol. Biotechnol. 2019. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.R.; Drews, A.; Kraume, M.; Shin, H.S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef]
- Gao, D.W.; Wen, Z.D.; Li, B.; Liang, H. Microbial community structure characteristics associated membrane fouling in A/O-MBR system. Bioresour. Technol. 2014, 154, 87–93. [Google Scholar] [CrossRef]
- Li, C.; Sun, W.; Lu, Z.; Ao, X.; Li, S. Ceramic nanocomposite membranes and membrane fouling: A review. Water Res. 2020, 175, 115674. [Google Scholar] [CrossRef]
- Maaz, M.; Yasin, M.; Aslam, M.; Kumar, G.; Atabani, A.E.; Idrees, M.; Anjum, F.; Jamil, F.; Ahmad, R.; Khan, A.L.; et al. Anaerobic membrane bioreactors for wastewater treatment: Novel configurations, fouling control and energy considerations. Bioresour. Technol. 2019, 283, 358–372. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, J.; Wei, Y.; Shen, P. Effects of ferric oxide on the microbial community and functioning during anaerobic digestion of swine manure. Bioresour. Technol. 2019, 287, 121393. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation-Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Ninomiya, Y.; Kimura, K.; Sato, T.; Kakuda, T.; Kaneda, M.; Hafuka, A.; Tsuchiya, T. High-flux operation of MBRs with ceramic flat-sheet membranes made possible by intensive membrane cleaning: Tests with real domestic wastewater under low-temperature conditions. Water Res. 2020, 181, 115881. [Google Scholar] [CrossRef]
- Homayoonfal, M.; Mehrnia, M.R.; Rahmani, S.; Mohades Mojtahedi, Y. Fabrication of alumina/polysulfone nanocomposite membranes with biofouling mitigation approach in membrane bioreactors. J. Ind. Eng. Chem. 2015, 22, 357–367. [Google Scholar] [CrossRef]
- Burman, I.; Sinha, A. Anaerobic hybrid membrane bioreactor for treatment of synthetic leachate: Impact of organic loading rate and sludge fractions on membrane fouling. Waste Manag. 2020, 108, 41–50. [Google Scholar] [CrossRef]
- Aslam, A.; Khan, S.J.; Shahzad, H.M.A. Impact of sludge recirculation ratios on the performance of anaerobic membrane bioreactor for wastewater treatment. Bioresour. Technol. 2019, 288, 121473. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, W.; De Costa, Y.G.; Zhuang, W.Q.; Yi, S. Assessing inorganic components of cake layer in A/O membrane bioreactor for physical-chemical treated tannery effluent. Chemosphere 2020, 250, 126220. [Google Scholar] [CrossRef]
- Chen, F.; Bi, X.; Ng, H.Y. Effects of bio-carriers on membrane fouling mitigation in moving bed membrane bioreactor. J. Membr. Sci. 2016, 499, 134–142. [Google Scholar] [CrossRef]
- Chen, C.; Guo, W.; Ngo, H.H.; Chang, S.W.; Duc Nguyen, D.; Dan Nguyen, P.; Bui, X.T.; Wu, Y. Impact of reactor configurations on the performance of a granular anaerobic membrane bioreactor for municipal wastewater treatment. Int. Biodeterior. Biodegrad. 2017, 121, 131–138. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, F.; Wang, D.; Jin, Y. Impact of reactor configuration on treatment performance and microbial diversity in treating high-strength dyeing wastewater: Anaerobic flat-sheet ceramic membrane bioreactor versus upflow anaerobic sludge blanket reactor. Bioresour. Technol. 2018, 269, 269–275. [Google Scholar] [CrossRef]
- Ibrahim, A.; Hiripitiyage, Y.; Peltier, E.; Sturm, B.S. Use of Halophilic Bacteria to Improve Aerobic Granular Sludge Integrity in Hypersaline Wastewaters. Environ. Eng. Sci. 2020, 37. [Google Scholar] [CrossRef]
- Wang, C.; Wu, H.; Wu, J.; Zhu, B.; Fan, W.; Lu, T.; Gao, Y.; Qi, W.; Niu, Q. Promoted biodegradation of para-ester wastewater by electrostimulated ZVI assisting novel UBF/ceramic membrane MBR and microbial community. J. Taiwan Inst. Chem. Eng. 2020, 113, 285–292. [Google Scholar] [CrossRef]
- Miura, Y.; Watanabe, Y.; Okabe, S. Membrane Biofouling in Pilot-Scale Membrane Bioreactors (MBRs) Treating Municipal Wastewater: Impact of Biofilm Formation. Environ. Sci. Technol. 2007, 41, 632–638. [Google Scholar] [CrossRef]
- Takahashi, S.; Miyahara, M.; Kouzuma, A.; Watanabe, K. Electricity generation from rice bran in microbial fuel cells. Bioresour. Bioprocess. 2016, 3, 50. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Zhang, J.; Tan, X.; McDougald, D.; Zhuang, G.; Fane, A.G.; Kjelleberg, S.; Cohen, Y.; Rice, S.A. Characterization of the archaeal community fouling a membrane bioreactor. J. Environ. Sci. 2015, 29, 115–123. [Google Scholar] [CrossRef]
- Calderon, K.; Rodelas, B.; Cabirol, N.; Gonzalez-Lopez, J.; Noyola, A. Analysis of microbial communities developed on the fouling layers of a membrane-coupled anaerobic bioreactor applied to wastewater treatment. Bioresour. Technol. 2011, 102, 4618–4627. [Google Scholar] [CrossRef]
- Lee, S.H.; Hong, T.I.; Kim, B.; Hong, S.; Park, H.D. Comparison of bacterial communities of biofilms formed on different membrane surfaces. World J. Microbiol. Biotechnol. 2014, 30, 777–782. [Google Scholar] [CrossRef]
- Tang, Z.; Lin, Z.; Wang, Y.; Zhao, P.; Kuang, F.; Zhou, J. Coupling of thermophilic biofilm-based systems and ozonation for enhanced organics removal from high-temperature pulping wastewater: Performance, microbial communities, and pollutant transformations. Sci. Total Environ. 2020, 714, 136802. [Google Scholar] [CrossRef]
- Gagliano, M.C.; Sudmalis, D.; Pei, R.; Temmink, H.; Plugge, C.M. Microbial Community Drivers in Anaerobic Granulation at High Salinity. Front. Microbiol. 2020, 11, 235. [Google Scholar] [CrossRef] [Green Version]
- Pakkulnan, R.; Anutrakunchai, C.; Kanthawong, S.; Taweechaisupapong, S.; Chareonsudjai, P.; Chareonsudjai, S. Extracellular DNA facilitates bacterial adhesion during Burkholderia pseudomallei biofilm formation. PLoS ONE 2019, 14, e0213288. [Google Scholar] [CrossRef]
- Bowman, J.P.; McCuaig, R.D. Biodiversity, community structural shifts, and biogeography of prokaryotes within Antarctic continental shelf sediment. Appl. Environ. Microbiol. 2003, 69, 2463–2483. [Google Scholar] [CrossRef] [Green Version]
- Dong, Q. Characterization of Anaerobic Membrane Bioreactors (AnMBR) Treating Municipal Wastewater. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2015. Available online: http://hdl.handle.net/10012/9179 (accessed on 31 March 2020).





| Resistance | Value |
|---|---|
| Rt (after 150 days operation) | 8.09 × 1012 |
| Rm | 2.02 × 1012 |
| Rr | 5.64 × 1012 |
| Rir | 0.42 × 1012 |
| Reactor Type | Reactor Volume (L) | Wastewater Type | Membrane Module | Membrane Material | Operational Mode | Cake Layer Resistance (Rr) | Fouling Control Method | Reference |
|---|---|---|---|---|---|---|---|---|
| AnCMBR | 3.6 | DWW | FS | Ceramic | -- | 95.2% | -- | [9] |
| MBMBR | 12.8 | DWW | FS | Ceramic | 84.8%/79.4% | bio carriers | [35] | |
| UAGB | 4 | DWW | HF | PVDF | 92% | [36] | ||
| CSTR | 5 | Synthetic DWW | HF | PVDF | 8 min permeation 2 min relaxation | 89–87.4% | hydrodynamic control | [33] |
| A/O MBR | 3 | tannery effluent | HF | PVC | suction mode of 10 min on/0.5 min off | 80% | -- | [34] |
| AnCMBR | 15 | DWW | T | Ceramic | AnCMBR | 69% | DWW | This study |
| Bacteria Phyla | Abundance (%) | Bacteria Phyla | Abundance (%) |
|---|---|---|---|
| Proteobacteria | 23.37 | Euryarchaeota | 1.87 |
| Bacteroidetes | 18.83 | Synergistetes | 1.17 |
| Chloroflexi | 18.26 | Actinobacteria | 1.09 |
| Firmicutes | 12.02 | Atribacteria | 0.92 |
| Thermotogae | 5.49 | Epsilonbacteraeota | 0.81 |
| unclassified_k__norank_d__Bacteria | 4.96 | Verrucomicrobia | 0.54 |
| Spirochaetes | 3.93 | Cloacimonetes | 0.19 |
| Patescibacteria | 3.58 | Armatimonadetes | 0.09 |
| others | 2.79 | Tenericutes | 0.01 |
| Archea Family | Abundance (%) | Archea Family | Abundance (%) |
|---|---|---|---|
| Methanosaetacea | 42.64 | Methanospirillaceae | 0.06 |
| Methanobacteriaceae | 22.40 | norank_o__norank_c__Micrarchaeia Methanomethylophilaceae | 0.05 0.03 |
| Methanomicrobiales | 14.29 | ||
| Methanomassiliicoccaceae | 8.37 | unclassified_p__Asgardaeota | 0.03 |
| Methanosarcinaceae | 7.75 | norank_o__norank_c__Bathyarchaeia | 0.02 |
| Methanofastidiosaceae | 2.28 | Methanomicrobiaceae | 0.009 |
| unclassified_k__norank_d__Archaea | 2.01 | Methanoregulaceae | 0.001 |
| Sample\Estimators | Sobs | Shannon | Simpson | Ace | Chao | Coverage |
|---|---|---|---|---|---|---|
| S1 | 291 | 3.462506 | 0.05852 | 303.9668 | 304 | 0.999618 |
| S2 | 664 | 4.245772 | 0.029664 | 779.7667 | 786.7662 | 0.997938 |
| D45 | 392 | 2.778037 | 0.182463 | 477.9779 | 502.5349 | 0.998259 |
| D90 | 685 | 3.876093 | 0.051158 | 880.5341 | 895.45 | 0.997332 |
| D150 | 768 | 4.338893 | 0.034709 | 959.76 | 997.2 | 0.997129 |
| Cake layer | 908 | 4.844364 | 0.018429 | 1081.579 | 1086.443 | 0.997169 |
| Permeate | 838 | 4.489069 | 0.029975 | 1073.885 | 1079.606 | 0.99488 |
| NaOCl | 789 | 4.225402 | 0.039877 | 1007.753 | 989.4492 | 0.994787 |
| Citric acid | 1092 | 4.18902 | 0.050877 | 1190.10 | 1164.675 | 0.996111 |
| Archaeal diversity | ||||||
| Sample\Estimators | Sobs | Shannon | Simpson | Ace | Chao | Coverage |
| S1 | 181 | 2.060121 | 0.278739 | 196.9229 | 191.3448 | 0.999497 |
| S2 | 435 | 3.234795 | 0.084348 | 474.861 | 462.8088 | 0.999022 |
| D45 | 224 | 1.357059 | 0.509039 | 314.5664 | 310.0588 | 0.997753 |
| D90 | 110 | 2.260526 | 0.168098 | 132.8407 | 131.4286 | 0.999592 |
| D150 | 81 | 1.562673 | 0.315953 | 137.2779 | 97.25 | 0.999607 |
| Cake layer | 107 | 1.819329 | 0.245727 | 190.9432 | 157.2143 | 0.999254 |
| Permeate | 96 | 1.095714 | 0.483232 | 127.0463 | 132.25 | 0.999545 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nilusha, R.T.; Wei, Y. New Insights into the Microbial Diversity of Cake Layer in Yttria Composite Ceramic Tubular Membrane in an Anaerobic Membrane Bioreactor (AnMBR). Membranes 2021, 11, 108. https://doi.org/10.3390/membranes11020108
Nilusha RT, Wei Y. New Insights into the Microbial Diversity of Cake Layer in Yttria Composite Ceramic Tubular Membrane in an Anaerobic Membrane Bioreactor (AnMBR). Membranes. 2021; 11(2):108. https://doi.org/10.3390/membranes11020108
Chicago/Turabian StyleNilusha, Rathmalgodage Thejani, and Yuansong Wei. 2021. "New Insights into the Microbial Diversity of Cake Layer in Yttria Composite Ceramic Tubular Membrane in an Anaerobic Membrane Bioreactor (AnMBR)" Membranes 11, no. 2: 108. https://doi.org/10.3390/membranes11020108
APA StyleNilusha, R. T., & Wei, Y. (2021). New Insights into the Microbial Diversity of Cake Layer in Yttria Composite Ceramic Tubular Membrane in an Anaerobic Membrane Bioreactor (AnMBR). Membranes, 11(2), 108. https://doi.org/10.3390/membranes11020108