Comparison of the Ammonia Trapping Performance of Different Gas-Permeable Tubular Membrane System Configurations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origin of the Slurry
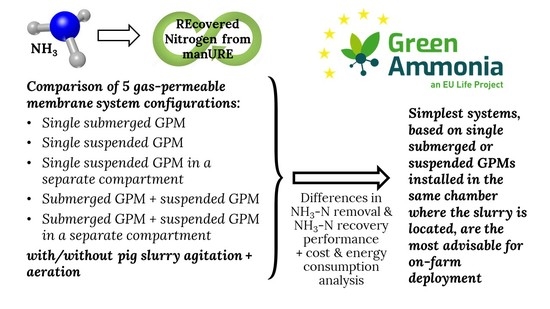
2.2. Experimental Design
- S1: Submerged GPM without slurry agitation or aeration.
- S2: Suspended GPM installed in the headspace of the treatment chamber without slurry agitation and aeration.
- S3: Suspended GPM installed in the headspace of a chamber attached to the treatment chamber where the slurry was without agitation or aeration.
- S4: Submerged and suspended GPMs in the same treatment chamber without agitation or aeration of the slurry.
- S5: Submerged GPM without slurry agitation or aeration and suspended GPM installed in the headspace of a chamber attached to the treatment chamber where the slurry was located.
- S6: GPM submerged with slurry agitation and aeration of 0.24 L air/(L slurry·min).
- S7: Suspended GPM installed in the headspace of the treatment chamber with slurry agitation and aeration of 0.24 L air/(L slurry·min).
- S8: Suspended GPM installed in the headspace of a chamber attached to the treatment chamber where the slurry was located with agitation and aeration of 0.24 L air/(L slurry·min).
- S9: Submerged and suspended GPMs in the same treatment chamber with slurry agitation and aeration of 0.24 L air/(L slurry·min).
- S10: Submerged GPM with slurry agitation and aeration of 0.24 L air/(L slurry·min) and suspended GPM installed in the headspace of a chamber attached to the treatment chamber where the slurry was located.
2.3. Analysis Methodology
2.4. Data Calculation
2.5. Statistical Analyses
2.6. Economic Analysis of the Different GPM Systems
- The annual production of pig slurry on the farm is 2795 m3 per year (i.e., 7.7 m3·d−1), resulting from 1300 animals producing 2.15 m3 slurry·year−1 each.
- Pig slurry contains between 931.6 and 1032.1 mg TAN·L−1 for each system evaluated.
- A TAN removal target for pig slurry of approximately 35% is proposed.
- TAN recovery efficiencies and TAN recovery rates at the end of the 7-day experimental period have been taken as a reference for each evaluated system.
- The cost of the membrane is 115 €·m−2 (1.88 €·m−1) [12] and a 10% replacement per year is considered.
- Annualized equipment costs are calculated assuming a useful life of 10 years and an interest rate of 8% [12].
- The amount of H2SO4 (98%) required to capture TAN ranges from 446.5 to 494.7 kg acid/kg recovered TAN for the systems evaluated in this study.
3. Results and Discussion
3.1. Nitrogen Removal from Pig Slurry
3.2. Nitrogen Recovery from Pig Slurry
3.3. N Flux Rates
3.4. Economic Cost Analysis of the Different GPM Systems
3.5. Practical Implications of the Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukhtar, S.; Mutlu, A.; Capareda, S.C.; Parnell, C.B. Seasonal and spatial variations of ammonia emissions from an open-lot dairy operation. J. Air Waste Manag. Assoc. 2008, 58, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.Q.; Cortus, E.L.; Heber, A.J. Improving ammonia emission modeling and inventories by data mining and intelligent interpretation of the national air emission monitoring study database. Atmosphere 2011, 2, 110–128. [Google Scholar] [CrossRef] [Green Version]
- Rothrock, M.J.; Szögi, A.A.; Vanotti, M.B. Recovery of ammonia from poultry litter using gas-permeable membranes. Trans. ASABE 2010, 53, 1267–1275. [Google Scholar] [CrossRef]
- Hristov, A.N.; Hanigan, M.; Cole, A.; Todd, R.; McAllister, T.A.; Ndegwa, P.M.; Rotz, A. Review: Ammonia emissions from dairy farms and beef feedlots. Can. J. Anim. Sci. 2011, 91, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Melse, R.W.; Ogink, N.W.M. Air scrubbing techniques for ammonia and odor reduction at livestock operations: Review of on-farm research in the Netherlands. Trans. Am. Soc. Agric. Eng. 2005, 48, 2303–2313. [Google Scholar] [CrossRef]
- Pagans, E.L.; Font, X.; Sánchez, A. Biofiltration for ammonia removal from composting exhaust gases. Chem. Eng. J. 2005, 113, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Rothrock, M.J.; Cook, K.L.; Warren, J.G.; Sistani, K. The effect of alum addition on microbial communities in poultry litter. Poult. Sci. 2008, 87, 1493–1503. [Google Scholar] [CrossRef]
- Mukhtar, S.; Samani Majd, A.M.; Borhan, M.S.; Beseda, J.F. An investigation of ammonia extraction from liquid manure using a gas-permeable membrane. In Proceedings of the 2011 ASABE Annual International Meeting, Louisville, KY, USA, 7–10 August 2011; paper number 1111178. American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2011. [Google Scholar] [CrossRef]
- Samani Majd, A.M.; Mukhtar, S. Ammonia recovery enhancement using a tubular gas-permeable membrane system in laboratory and field-scale studies. Trans. ASABE 2013, 56, 1951–1958. [Google Scholar] [CrossRef]
- Sürmeli, R.Ö.; Bayrakdar, A.; Çalli, B. Ammonia recovery from chicken manure digestate using polydimethylsiloxane membrane contactor. J. Clean. Prod. 2018, 191, 99–104. [Google Scholar] [CrossRef]
- Vanotti, M.B.; Szogi, A.A. Use of gas-permeable membranes for the removal and recovery of ammonia from high strength livestock wastewater. Proc. Water Environ. Fed. 2012, 2011, 659–667. [Google Scholar] [CrossRef]
- Dube, P.J.; Vanotti, M.B.; Szogi, A.A.; García-González, M.C. Enhancing recovery of ammonia from swine manure anaerobic digester effluent using gas-permeable membrane technology. Waste Manag. 2016, 49, 372–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothrock, M.J.; Szögi, A.A.; Vanotti, M.B. Recovery of ammonia from poultry litter using flat gas permeable membranes. Waste Manag. 2013, 33, 1531–1538. [Google Scholar] [CrossRef]
- Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Martín-Ramos, P. Pilot plant for the capture of ammonia from the atmosphere of pig and poultry farms using gas-permeable membrane technology. Membranes 2021, 11, 859. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.T.; Hwang, Y.H.; Shin, H.S. Application of PTFE membrane for ammonia removal in a membrane contactor. Water Sci. Technol. 2011, 63, 2944–2948. [Google Scholar] [CrossRef] [PubMed]
- Garcia-González, M.C.; Vanotti, M.B. Recovery of ammonia from swine manure using gas-permeable membranes: Effect of waste strength and pH. Waste Manag. 2015, 38, 455–461. [Google Scholar] [CrossRef]
- Fillingham, M.; VanderZaag, A.C.; Singh, J.; Burtt, S.; Crolla, A.; Kinsley, C.; MacDonald, J.D. Characterizing the performance of gas-permeable membranes as an ammonia recovery strategy from anaerobically digested dairy manure. Membranes 2017, 7, 59. [Google Scholar] [CrossRef] [Green Version]
- Nagy, J.; Kaljunen, J.; Toth, A.J. Nitrogen recovery from wastewater and human urine with hydrophobic gas separation membrane: Experiments and modelling. Chem. Pap. 2019, 73, 1903–1915. [Google Scholar] [CrossRef] [Green Version]
- Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Vanotti, M.B.; Martín-Ramos, P. Effect of acid flow rate, membrane surface area, and capture solution on the effectiveness of suspended GPM systems to recover ammonia. Membranes 2021, 11, 538. [Google Scholar] [CrossRef]
- Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Martín-Ramos, P. Evaluation of different capture solutions for ammonia recovery in suspended gas permeable membrane systems. Membranes 2022, 12, 572. [Google Scholar] [CrossRef]
- González-García, I.; Riaño, B.; Molinuevo-Salces, B.; Vanotti, M.B.; García-González, M.C. Improved anaerobic digestion of swine manure by simultaneous ammonia recovery using gas-permeable membranes. Water Res. 2021, 190, 116789. [Google Scholar] [CrossRef]
- García-González, M.C.; Vanotti, M.B.; Szogi, A.A. Recovery of ammonia from swine manure using gas-permeable membranes: Effect of aeration. J. Environ. Manag. 2015, 152, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Riaño, B.; Molinuevo-Salces, B.; Vanotti, M.B.; García-González, M.C. Application of gas-permeable membranes for-semi-continuous ammonia recovery from swine manure. Environments 2019, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Ebnesajjad, S. Expanded PTFE Applications Handbook: Technology, Manufacturing and Applications; Andrew, W., Ed.; Elsevier Science: New York, NY, USA, 2016; ISBN 1437778569/9781437778564. [Google Scholar]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Washington DC, USA, 2017. [Google Scholar]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- R Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Daguerre-Martini, S.; Vanotti, M.B.; Rodriguez-Pastor, M.; Rosal, A.; Moral, R. Nitrogen recovery from wastewater using gas-permeable membranes: Impact of inorganic carbon content and natural organic matter. Water Res. 2018, 137, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Oliveira Filho, J.D.S.; Daguerre-Martini, S.; Vanotti, M.B.; Saez-Tovar, J.; Rosal, A.; Perez-Murcia, M.D.; Bustamante, M.A.; Moral, R. Recovery of ammonia in raw and co-digested swine manure using gas-permeable membrane technology. Front. Sustain. Food Syst. 2018, 2, 30. [Google Scholar] [CrossRef] [Green Version]
- García-González, M.C.; Vanotti, M.B. Nitrogen recovery from liquid manure using gas-permeable membranes: Effect of wastewater strength and pH control. In Proceedings of the 2014 ASABE and CSBE/SCGAB Annual International Meeting, Montreal, QC, Canada, 13–16 July 2014; American Society of Agricultural and Biological Engineers: Montreal, QC, Canada, 2014; Volume 7004. [Google Scholar]
- Vanotti, M.B.; Dube, P.J.; Szogi, A.A.; García-González, M.C. Recovery of ammonia and phosphate minerals from swine wastewater using gas-permeable membranes. Water Res. 2017, 112, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Serra-Toro, A.; Astals, S.; Madurga, S.; Mata-Álvarez, J.; Mas, F.; Dosta, J. Ammoniacal nitrogen recovery from pig slurry using a novel hydrophobic/hydrophilic selective membrane. J. Environ. Chem. Eng. 2022, 10, 108434. [Google Scholar] [CrossRef]
- Zarebska, A.; Nieto, D.R.; Christensen, K.V.; Norddahl, B. Ammonia recovery from agricultural wastes by membrane distillation: Fouling characterization and mechanism. Water Res. 2014, 56, 1–10. [Google Scholar] [CrossRef]
- González-García, I.; Oliveira, V.; Molinuevo-Salces, B.; García-González, M.C.; Dias-Ferreira, C.; Riaño, B. Two-phase nutrient recovery from livestock wastewaters combining novel membrane technologies. Biomass Convers. Biorefinery 2022, 12, 4563–4574. [Google Scholar] [CrossRef]
- Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Martín-Ramos, P. Reduction of ammonia emissions from laying hen manure in a closed composting process using gas-permeable membrane technology. Agronomy 2021, 11, 2384. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Riaño, B.; Vanotti, M.B.; Hernández-González, D.; García-González, M.C. Pilot-scale demonstration of membrane-based nitrogen recovery from swine manure. Membranes 2020, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- United Nations Economic and Social Council. ECE/EB.AIR/120: Guidance Document on Preventing and Abating Ammonia Emissions from Agricultural Sources; United Nations Economic and Social Council, Economic Commission for Europe, Executive Body for the Convention on Long-Range Transboundary Air Pollution: Geneva, Switzerland, 2014. Available online: https://unece.org/fileadmin/DAM/env/documents/2012/EB/ECE_EB.AIR_120_ENG.pdf (accessed on 3 November 2022).
- Zarebska, A.; Romero Nieto, D.; Christensen, K.V.; Fjerbæk Søtoft, L.; Norddahl, B. Ammonium fertilizers production from manure: A critical review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1469–1521. [Google Scholar] [CrossRef]
- De Vrieze, J.; Colica, G.; Pintucci, C.; Sarli, J.; Pedizzi, C.; Willeghems, G.; Bral, A.; Varga, S.; Prat, D.; Peng, L.; et al. Resource recovery from pig manure via an integrated approach: A technical and economic assessment for full-scale applications. Bioresour. Technol. 2019, 272, 582–593. [Google Scholar] [CrossRef] [PubMed]

| Conditions | GPM System | Initial NH3-N Mass (mg) | NH3-N Removed (mg) | NH3-NRecovered (mg) | NH3-N Removal (%) | NH3-N Recovered (%) | N Flux (g·m−2·d−1) |
|---|---|---|---|---|---|---|---|
| Without agitation + aeration | S1 | 4125 ± 227 | 1599 ± 136 b | 1414 ± 21 cd | 39 | 88 | 12.4 ± 0.2 cd |
| S2 | 4308 ± 47 | 1567 ± 96 b | 1083 ± 8 e | 36 | 69 | 9.5 ± 1.7 e | |
| S3 | 4340 ± 16 | 2680 ± 119 a | 1453 ± 111 c | 62 | 54 | 12.7 ± 1.0 c | |
| S4 | 4125 ± 227 | 2710 ± 172 a | 2361 ± 61 a | 66 | 87 | 10.3 ± 0.3 cde | |
| S5 | 4006 ± 116 | 2692 ± 241 a | 2278 ± 41 a | 67 | 85 | 10.0 ± 0.2 de | |
| With agitation + aeration | S6 | 4125 ± 227 | 2545 ± 66 a | 2328 ± 10 a | 62 | 91 | 20.4 ± 0.0 a |
| S7 | 4122 ± 46 | 2696± 142 a | 1918 ± 197 b | 65 | 71 | 16.8 ± 0.1 b | |
| S8 | 4006 ± 116 | 2901 ± 327 a | 1272 ± 20 de | 72 | 44 | 11.4 ± 0.2 cde | |
| S9 | 4437 ± 20 | 3211 ± 29 a | 2545 ± 259 a | 72 | 79 | 11.1 ± 0.2 cde | |
| S10 | 4006 ± 116 | 2824 ± 254 a | 2434 ± 2 a | 70 | 86 | 10.6 ± 0.0 de |
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Membrane cost (€/m) | 1.88 | |||||||||
| Membrane cost (€/m2) | 115.24 | |||||||||
| Investment | ||||||||||
| Membrane initial cost (€) | 25,686 | 32,573 | 24,547 | 30,923 | 28,774 | 15,690 | 17,625 | 25,240 | 28,719 | 27,145 |
| Total initial investment (including membranes and other equipment) (€) | 44,478 | 51,365 | 54,701 | 58,577 | 58,928 | 38,697 | 40,632 | 50,234 | 60,588 | 61,514 |
| Annualized costs 8% interest, 10-year life (€/year) | 6629 | 7655 | 8152 | 8730 | 8782 | 5767 | 6055 | 7486 | 9029 | 9167 |
| Operating Costs | ||||||||||
| Membrane replacement 10% (€) | 2569 | 3257 | 2455 | 3092 | 2877 | 1569 | 1763 | 2524 | 2872 | 2715 |
| Chemicals (€) | 143 | 139 | 140 | 143 | 130 | 143 | 133 | 130 | 144 | 130 |
| Power (€) | 1610 | 1610 | 3329 | 3329 | 3329 | 6537 | 6537 | 6811 | 8256 | 8256 |
| Total annualized costs (€/year) | 10,950 | 12,661 | 14,076 | 15,294 | 15,118 | 14,017 | 14,488 | 16,951 | 20,301 | 20,268 |
| Revenue for the sale of the fertilizer product (€/year) | 1049 | 1019 | 1027 | 1049 | 948 | 1049 | 975 | 948 | 1050 | 948 |
| Net annual cost (€/year) | 9901 | 11,642 | 13,049 | 14,245 | 14,170 | 12,967 | 13,513 | 16,003 | 19,251 | 19,320 |
| Cost per N recovered (€/kg N) | 9.8 | 11.9 | 13.2 | 14.1 | 15.5 | 12.9 | 14.4 | 17.6 | 19.1 | 21.2 |
| Cost per treated slurry (€/m3) | 3.5 | 4.2 | 4.7 | 5.1 | 5.1 | 4.6 | 4.8 | 5.7 | 6.9 | 6.9 |
| Net cost (€/(place·year)) | 7.6 | 9.0 | 10.0 | 11.0 | 10.9 | 10.0 | 10.4 | 12.3 | 14.8 | 14.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Herranz, M.; Sánchez-Báscones, M.; García-González, M.C.; Martín-Ramos, P. Comparison of the Ammonia Trapping Performance of Different Gas-Permeable Tubular Membrane System Configurations. Membranes 2022, 12, 1104. https://doi.org/10.3390/membranes12111104
Soto-Herranz M, Sánchez-Báscones M, García-González MC, Martín-Ramos P. Comparison of the Ammonia Trapping Performance of Different Gas-Permeable Tubular Membrane System Configurations. Membranes. 2022; 12(11):1104. https://doi.org/10.3390/membranes12111104
Chicago/Turabian StyleSoto-Herranz, María, Mercedes Sánchez-Báscones, María Cruz García-González, and Pablo Martín-Ramos. 2022. "Comparison of the Ammonia Trapping Performance of Different Gas-Permeable Tubular Membrane System Configurations" Membranes 12, no. 11: 1104. https://doi.org/10.3390/membranes12111104
APA StyleSoto-Herranz, M., Sánchez-Báscones, M., García-González, M. C., & Martín-Ramos, P. (2022). Comparison of the Ammonia Trapping Performance of Different Gas-Permeable Tubular Membrane System Configurations. Membranes, 12(11), 1104. https://doi.org/10.3390/membranes12111104