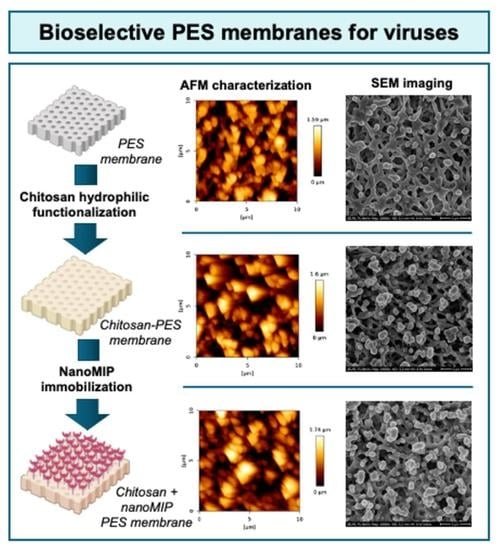
Bioselective PES Membranes Based on Chitosan Functionalization and Virus-Imprinted NanoMIPs for Highly Efficient Separation of Human Pathogenic Viruses from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Membrane Functionalization with Chitosan
2.3. NanoMIP Immobilization
2.4. Characterization of Membranes
2.4.1. Contact Angle Measurements and Swelling Behavior
2.4.2. AFM Characterization
2.4.3. SEM Imaging
2.5. Membrane Performance Experiments
3. Results and Discussion
3.1. Contact Angle Measurements and Swelling Behavior
3.2. AFM Characterization
3.3. SEM Imaging of PES Membranes
3.4. Filtration Experiments
3.4.1. Permeation Flux
3.4.2. Membrane Fouling Studies
3.4.3. Filtration Studies and Uptake Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kroehler, C.J. Potable Water Quality Standards and Regulations: A Historical and World Overview. In Potable Water; Younos, T., Grady, C.A., Eds.; Springer International Publishing: Cham, Switzerland, 2014; Volume 30, pp. 1–36. ISBN 9783319065625. [Google Scholar]
- Griffiths, J.K. Waterborne Diseases. In International Encyclopedia of Public Health; Elsevier: Amsterdam, The Netherlands, 2017; pp. 388–401. ISBN 9780128037089. [Google Scholar]
- University of Delaware. New Technology Removes Viruses From Drinking Water. ScienceDialy. 2007. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 17 July 2022).
- Fong, T.-T.; Lipp, E.K. Enteric Viruses of Humans and Animals in Aquatic Environments: Health Risks, Detection, and Potential Water Quality Assessment Tools. Microbiol. Mol. Biol. Rev. 2005, 69, 357–371. [Google Scholar] [CrossRef] [Green Version]
- Kocwa-Haluch, R. Waterborne enteroviruses as a hazard for human health. Pol. J. Environ. Stud. 2001, 10, 485–488. [Google Scholar]
- Haas, C.N.; Rose, J.B.; Gerba, C.; Regli, S. Risk Assessment of Virus in Drinking Water. Risk Anal. 1993, 13, 545–552. [Google Scholar] [CrossRef]
- Haramoto, E.; Kitajima, M.; Hata, A.; Torrey, J.R.; Masago, Y.; Sano, D.; Katayama, H. A Review on Recent Progress in the Detection Methods and Prevalence of Human Enteric Viruses in Water. Water Res. 2018, 135, 168–186. [Google Scholar] [CrossRef]
- Mackowiak, M.; Leifels, M.; Hamza, I.A.; Jurzik, L.; Wingender, J. Distribution of Escherichia Coli, Coliphages and Enteric Viruses in Water, Epilithic Biofilms and Sediments of an Urban River in Germany. Sci. Total Environ. 2018, 626, 650–659. [Google Scholar] [CrossRef]
- Ganesh, A.; Lin, J. Waterborne Human Pathogenic Viruses of Public Health Concern. Int. J. Environ. Health Res. 2013, 23, 544–564. [Google Scholar] [CrossRef]
- Seidel, M.; Jurzik, L.; Brettar, I.; Höfle, M.G.; Griebler, C. Microbial and Viral Pathogens in Freshwater: Current Research Aspects Studied in Germany. Environ. Earth Sci. 2016, 75, 1384. [Google Scholar] [CrossRef]
- Hmaied, F.; Keskes, S.; Jebri, S.; Amri, I.; Yahya, M.; Loisy-Hamon, F.; Lebeau, B.; Hamdi, M. Removal of Rotavirus and Bacteriophages by Membrane Bioreactor Technology from Sewage. Curr. Microbiol. 2015, 71, 540–545. [Google Scholar] [CrossRef]
- Kwarciak-Kozlowska, A.; Wlodarczyk, R. Chapter 5—Treatment of Waterborne Pathogens by Microfiltration. In Waterborne Pathogens; Vara Prasad, M.N., Grobelak, A., Eds.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 81–103. ISBN 9780128187838. [Google Scholar]
- Ayode Otitoju, T.; Latif Ahmad, A.; Seng Ooi, B. Recent Advances in Hydrophilic Modification and Performance of Polyethersulfone (PES) Membrane via Additive Blending. RSC Adv. 2018, 8, 22710–22728. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Lu, J.; Wu, Y.; Meng, M.; Yu, C.; Sun, C.; Chen, M.; Da, Z.; Yan, Y. Antifouling Molecularly Imprinted Membranes for Pretreatment of Milk Samples: Selective Separation and Detection of Lincomycin. Food Chem. 2020, 333, 127477. [Google Scholar] [CrossRef]
- Altintas, Z.; Gittens, M.; Pocock, J.; Tothill, I.E. Biosensors for waterborne viruses: Detection and removal. Biochimie 2015, 115, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A.M.; Adam, M.R.; Mohamad Kamal, S.N.E.A.; Jaafar, J.; Othman, M.H.D.; Ismail, A.F.; Aziz, F.; Yusof, N.; Bilad, M.R.; Mohamud, R.; et al. A Review of the Potential of Conventional and Advanced Membrane Technology in the Removal of Pathogens from Wastewater. Sep. Purif. Technol. 2022, 286, 120454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, Y.; Gao, A.; Zhang, Q.; Cui, J.; Zhao, S.; Zhan, X.; Yan, Y. Bio-Inspired Underwater Superoleophobic PVDF Membranes for Highly-Efficient Simultaneous Removal of Insoluble Emulsified Oils and Soluble Anionic Dyes. Chem. Eng. J. 2019, 369, 576–587. [Google Scholar] [CrossRef]
- Altintas, Z. Advanced imprinted materials for virus monitoring. In Advanced Molecularly Imprinted Materials; Tiwari, A., Uzun, L., Eds.; WILEY-Scrivener: Beverly, MA, USA, 2016; pp. 389–412. ISBN 9781119336297. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Tharpa, K.; Dima, Ş.-O. Molecularly Imprinted Membranes: Past, Present, and Future. Chem. Rev. 2016, 116, 11500–11528. [Google Scholar] [CrossRef]
- Altintas, Z.; Gittens, M.; Guerreiro, A.; Thompson, K.-A.; Walker, J.; Piletsky, S.; Tothill, I.E. Detection of Waterborne Viruses Using High Affinity Molecularly Imprinted Polymers. Anal. Chem. 2015, 87, 6801–6807. [Google Scholar] [CrossRef]
- Altintas, Z.; Pocock, J.; Thompson, K.-A.; Tothill, I.E. Comparative Investigations for Adenovirus Recognition and Quantification: Plastic or Natural Antibodies? Biosens. Bioelectron. 2015, 74, 996–1004. [Google Scholar] [CrossRef]
- Bajaj, A.; Trimpert, J.; Abdulhalim, I.; Altintas, Z. Synthesis of molecularly imprinted polymer nanoparticles for SARS-CoV-2 virus detection using surface plasmon resonance. Chemosensors 2022, 10, 459. [Google Scholar] [CrossRef]
- Zaidi, S.A. An Overview of Bio-Inspired Intelligent Imprinted Polymers for Virus Determination. Biosensors 2021, 11, 89. [Google Scholar] [CrossRef]
- Smolinska-Kempisty, K.; Wolska, J.; Bryjak, M. Molecularly Imprinting Microfiltration Membranes Able to Absorb Diethyl Phthalate from Water. Membranes 2022, 12, 503. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Partridge, A.; Wu, Y. Improving Nanoparticle-Enhanced Surface Plasmon Resonance Detection of Small Molecules by Reducing Steric Hindrance via Molecular Linkers. Talanta 2019, 198, 350–357. [Google Scholar] [CrossRef]
- Geens, J.; Van der Bruggen, B.; Vandecasteele, C. Characterisation of the Solvent Stability of Polymeric Nanofiltration Membranes by Measurement of Contact Angles and Swelling. Chem. Eng. Sci. 2004, 59, 1161–1164. [Google Scholar] [CrossRef]
- Mkhize, D.S.; Nyoni, H.; Quinn, L.P.; Mamba, B.B.; Msagati, T.A.M. Molecularly Imprinted Membranes (MIMs) for Selective Removal of Polychlorinated Biphenyls (PCBs) in Environmental Waters: Fabrication and Characterization. Environ. Sci. Pollut. Res. 2017, 24, 11694–11707. [Google Scholar] [CrossRef] [PubMed]
- Boributh, S.; Chanachai, A.; Jiraratananon, R. Modification of PVDF Membrane by Chitosan Solution for Reducing Protein Fouling. J. Membr. Sci. 2009, 342, 97–104. [Google Scholar] [CrossRef]
- Johannsmann, D.; Langhoff, A.; Leppin, C. Studying Soft Interfaces with Shear Waves: Principles and Applications of the Quartz Crystal Microbalance (QCM). Sensors 2021, 21, 3490. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Z.; Chianella, I.; Da Ponte, G.; Paulussen, S.; Gaeta, S.; Tothill, I.E. Development of Functionalized Nanostructured Polymeric Membranes for Water Purification. Chem. Eng. J. 2016, 300, 358–366. [Google Scholar] [CrossRef]











| Sample | Wdry [g] | Wwet [g] | Degree of Swelling |
|---|---|---|---|
| Bare PES membrane | 52.5 | 109.7 | 1.08 |
| Chitosan functionalized membrane | 54.1 | 115.6 | 1.13 |
| Chitosan + nanoMIP functionalized membrane | 55.3 | 125.3 | 1.26 |
| Size [µm] | Bare PES Membrane | Chitosan Functionalized Membrane | Chitosan + nanoMIP Functionalized Membrane | |||
|---|---|---|---|---|---|---|
| Average RMS [nm] | Standard Deviation [nm] | Average RMS [nm] | Standard Deviation [nm] | Average RMS [nm] | Standard Deviation [nm] | |
| 50 × 50 | 391.89 | 24.29 | 416.58 | 24.31 | 448.67 | 26.65 |
| 10 × 10 | 300.58 | 18.09 | 351.46 | 20.21 | 402.87 | 19.36 |
| 3 × 3 | 188.68 | 12.49 | 221.26 | 9.40 | 229.55 | 21.26 |
| Sample | Vloading solution [mL] | Permeation Flux [L m−2 h−1] | %Volume Recovered from Loading Solution |
|---|---|---|---|
| Bare PES membrane | 5 | 730.76 | 80.00 |
| 10 | 991.63 | 87.00 | |
| Chitosan functionalized membrane | 5 | 1225.19 | 96.00 |
| 10 | 1090.82 | 94.00 | |
| Chitosan + nanoMIP functionalized membrane | 5 | 1118.06 | 86.00 |
| 10 | 1149.27 | 88.00 |
| Sample | Filtration Order | Vloading solution [mL] | Permeation Flux [L m−2 h−1] | FDR [%] |
|---|---|---|---|---|
| Bare PES membrane | First | 10 | 1490.97 | - |
| Second | 10 | 1031.52 | 30.81 | |
| Third | 10 | 848.49 | 17.74 | |
| Chitosan functionalized PES membrane | First | 10 | 1225.18 | - |
| Second | 10 | 966.14 | 21.14 | |
| Third | 10 | 842.38 | 12.80 |
| Sample | Percentage of Loading Concentration | Uptake Capacity [%] | NanoMIP Distribution Density [µg cm−2] |
|---|---|---|---|
| Bare PES membrane Vloading solution = 5 mL | 1.07 × 10−5 | 99.99 | no nanoMIPs |
| Bare PES membrane Vloading solution = 10 mL | 79.04 | 20.95 | no nanoMIPs |
| Chitosan + nanoMIP functionalized membrane Vloading solution = 5 mL | 2.05 × 10−8 | 99.99 | 5.1 |
| Chitosan + nanoMIP functionalized membrane Vloading solution = 10 mL | 0.008 | 99.99 | 5.1 |
| Sample | Virus Recovery in Buffer after Regeneration [%] |
|---|---|
| First filtration after regeneration with HCl | 0.003 |
| Second filtration after regeneration with HCl | 0.0003 |
| First filtration after regeneration with cold ACN | 2.12 × 10−5 |
| First filtration after regeneration with hot ACN | 1.36 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivares Moreno, C.A.; Altintas, Z. Bioselective PES Membranes Based on Chitosan Functionalization and Virus-Imprinted NanoMIPs for Highly Efficient Separation of Human Pathogenic Viruses from Water. Membranes 2022, 12, 1117. https://doi.org/10.3390/membranes12111117
Olivares Moreno CA, Altintas Z. Bioselective PES Membranes Based on Chitosan Functionalization and Virus-Imprinted NanoMIPs for Highly Efficient Separation of Human Pathogenic Viruses from Water. Membranes. 2022; 12(11):1117. https://doi.org/10.3390/membranes12111117
Chicago/Turabian StyleOlivares Moreno, Carmen Andreina, and Zeynep Altintas. 2022. "Bioselective PES Membranes Based on Chitosan Functionalization and Virus-Imprinted NanoMIPs for Highly Efficient Separation of Human Pathogenic Viruses from Water" Membranes 12, no. 11: 1117. https://doi.org/10.3390/membranes12111117
APA StyleOlivares Moreno, C. A., & Altintas, Z. (2022). Bioselective PES Membranes Based on Chitosan Functionalization and Virus-Imprinted NanoMIPs for Highly Efficient Separation of Human Pathogenic Viruses from Water. Membranes, 12(11), 1117. https://doi.org/10.3390/membranes12111117