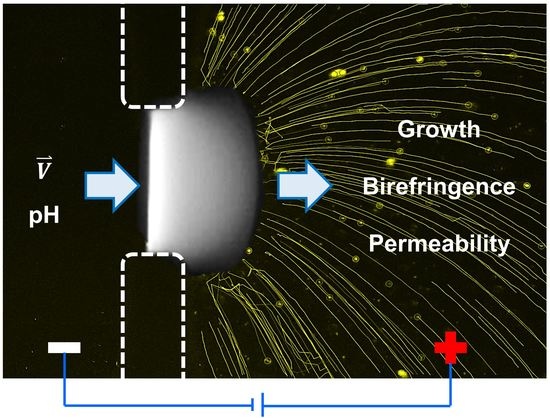
Programmable Physical Properties of Freestanding Chitosan Membranes Electrofabricated in Microfluidics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microfluidic Platform Preparation
2.3. Fabrication of Chitosan Membranes
2.4. Membrane Growth Rate
2.5. Permeability Test
2.6. Birefringence and Parallelism Index Analysis
2.7. Statistical Analysis
3. Results
3.1. Membrane Growth Rate
3.2. Membrane Permeability
3.3. Membrane Birefringence
3.4. High Throughput Electrofabrication of Chitosan Membranes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- He, Y.; Pan, J.; Wu, L.; Ge, L.; Xu, T. Facile preparation of 1,8-Diazabicyclo[5.4.0]undec-7-ene based high performance anion exchange membranes for diffusion dialysis applications. J. Membr. Sci. 2015, 491, 45–52. [Google Scholar] [CrossRef]
- Cardoso, S.d.A.; Macêdo, E.N.; Quaresma, J.N.N. Improved lumped solutions for mass transfer analysis in membrane separation process of metals. Int. J. Heat Mass Transf. 2014, 68, 599–611. [Google Scholar] [CrossRef]
- Wei, H.; Chueh, B.-H.; Wu, H.; Hall, E.W.; Li, C.-W.; Schirhagl, R.; Lin, J.-M.; Zare, R.N. Particle sorting using a porous membrane in a microfluidic device. Lab Chip 2011, 11, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Lu, W.-Q. A numerical simulation for mass transfer through the porous membrane of parallel straight channels. Int. J. Heat Mass Transf. 2010, 53, 2404–2413. [Google Scholar] [CrossRef]
- Li, D.; Choi, H.; Cho, S.; Jeong, S.; Jin, Z.; Lee, C.; Ko, S.Y.; Park, J.-O.; Park, S. A hybrid actuated microrobot using an electromagnetic field and flagellated bacteria for tumor-targeting therapy. Biotechnol. Bioeng. 2015, 112, 1623–1631. [Google Scholar] [CrossRef]
- Sreenivasan, R.; Bassett, E.K.; Hoganson, D.M.; Vacanti, J.P.; Gleason, K.K. Ultra-thin, gas permeable free-standing and composite membranes for microfluidic lung assist devices. Biomaterials 2011, 32, 3883–3889. [Google Scholar] [CrossRef]
- de Jong, J.; Lammertink, R.G.; Wessling, M. Membranes and microfluidics: A review. Lab Chip 2006, 6, 1125–1139. [Google Scholar] [CrossRef]
- Wang, P.-C.; DeVoe, D.L.; Lee, C.S. Integration of polymeric membranes with microfluidic networks for bioanalytical applications. Electrophoresis 2001, 22, 3857–3867. [Google Scholar] [CrossRef]
- Moskvin, L.; Nikitina, T. Membrane Methods of Substance Separation in Analytical Chemistry. J. Anal. Chem. 2004, 59, 2–16. [Google Scholar] [CrossRef]
- Ly, K.L.; Hu, P.; Pham, L.H.P.; Luo, X. Flow-assembled chitosan membranes in microfluidics: Recent advances and applications. J. Mater. Chem. B 2021, 9, 3258–3283. [Google Scholar] [CrossRef]
- Rosella, E.; Jia, N.; Mantovani, D.; Greener, J. A microfluidic approach for development of hybrid collagen-chitosan extracellular matrix-like membranes for on-chip cell cultures. J. Mater. Sci. Technol. 2020, 63, 54–61. [Google Scholar] [CrossRef]
- Wang, J.; Bettinger, C.; Langer, R.; Borenstein, J. Biodegradable microfluidic scaffolds for tissue engineering from amino alcohol-based poly(ester amide) elastomers. Organogenesis 2014, 6, 212–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, C.M.; de Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A guide to the organ-on-a-chip. Nat. Rev. Methods Prim. 2022, 2, 33. [Google Scholar] [CrossRef]
- Correa, S.O.; Luo, X.; Raub, C.B. Microfluidic fabrication of stable collagen microgels with aligned microstructure using flow-driven co-deposition and ionic gelation. J. Micromechanics Microengineering 2020, 30, 085002. [Google Scholar] [CrossRef]
- Vedula, S.S.; Yadav, G.D. Chitosan-based membranes preparation and applications: Challenges and opportunities. J. Indian Chem. Soc. 2021, 98, 100017. [Google Scholar] [CrossRef]
- Liu, Z.-B.; Zhang, Y.; Yu, J.-J.; Mak, A.F.-T.; Li, Y.; Yang, M. A microfluidic chip with poly(ethylene glycol) hydrogel microarray on nanoporous alumina membrane for cell patterning and drug testing. Sens. Actuators B Chem. 2010, 143, 776–783. [Google Scholar] [CrossRef]
- Sun, Y.-M.; Wang, W.; Wei, Y.-Y.; Deng, N.-N.; Liu, Z.; Ju, X.-J.; Xie, R.; Chu, L.-Y. In situ fabrication of a temperature- and ethanol-responsive smart membrane in a microchip. Lab Chip 2014, 14, 2418–2427. [Google Scholar] [CrossRef]
- Moorthy, J.; Beebe, D.J. In situ fabricated porous filters for microsystems. Lab Chip 2003, 3, 62–66. [Google Scholar] [CrossRef]
- Nair, G.; Gargiuli, J.; Raveendran, S.; Rong, Z.; Shapiro, E.; Drikakis, D.; Vadgama, P. In Situ Fabrication of Cross-Linked Protein Membranes by Using Microfluidics. Chembiochem A Eur. J. Chem. Biol. 2006, 7, 1683–1689. [Google Scholar] [CrossRef]
- Luo, X.; Vo, T.; Jambi, F.; Pham, P.; Choy, J.S. Microfluidic partition with in situ biofabricated semipermeable biopolymer membranes for static gradient generation. Lab Chip 2016, 16, 3815–3823. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Suginta, W.; Khunkaewla, P.; Schulte, A. Electrochemical biosensor applications of polysaccharides chitin and chitosan. Chem. Rev. 2013, 113, 5458–5479. [Google Scholar] [CrossRef] [PubMed]
- Doan, V.K.; Ly, K.L.; Tran, N.M.-P.; Ho, T.P.-T.; Ho, M.H.; Dang, N.T.-N.; Chang, C.-C.; Nguyen, H.T.-T.; Ha, P.T.; Tran, Q.N.; et al. Characterizations and Antibacterial Efficacy of Chitosan Oligomers Synthesized by Microwave-Assisted Hydrogen Peroxide Oxidative Depolymerization Method for Infectious Wound Applications. Materials 2021, 14, 4475. [Google Scholar] [CrossRef] [PubMed]
- Tibbe, M.P.; Leferink, A.M.; van den Berg, A.; Eijkel, J.C.T.; Segerink, L.I. Microfluidic Gel Patterning Method by Use of a Temporary Membrane for Organ-on-Chip Applications. Adv. Mater. Technol. 2018, 3, 1700200. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.; Ly, K.L.; Pham, L.P.H.; Pottash, A.E.; Sheridan, K.; Wu, H.-C.; Tsao, C.-Y.; Quan, D.; Bentley, W.E.; Rubloff, G.W.; et al. Bacterial chemotaxis in static gradients quantified in a biopolymer membrane-integrated microfluidic platform. Lab Chip 2022, 22, 3203–3216. [Google Scholar] [CrossRef]
- Vo, T.; Shah, S.B.; Choy, J.S.; Luo, X. Chemotropism among populations of yeast cells with spatiotemporal resolution in a biofabricated microfluidic platform. Biomicrofluidics 2020, 14, 014108. [Google Scholar] [CrossRef]
- Luo, X.; Wu, H.-C.; Tsao, C.-Y.; Cheng, Y.; Betz, J.; Payne, G.F.; Rubloff, G.W.; Bentley, W.E. Biofabrication of stratified biofilm mimics for observation and control of bacterial signaling. Biomaterials 2012, 33, 5136–5143. [Google Scholar] [CrossRef]
- Pham, P.L.H.; Rooholghodos, S.A.; Choy, J.S.; Luo, X. Constructing Synthetic Ecosystems with Biopolymer Fluitrodes. Adv. Biosyst. 2018, 2, 1700180. [Google Scholar] [CrossRef]
- Pham, L.H.P.; Colon-Ascanio, M.; Ou, J.; Ly, K.; Hu, P.; Choy, J.S.; Luo, X. Probing mutual interactions between Pseudomonas aeruginosa and Candida albicans in a biofabricated membrane-based microfluidic platform. Lab Chip 2022, 22, 4349–4358. [Google Scholar] [CrossRef]
- Wu, S.; Kim, E.; Chen, C.-y.; Li, J.; VanArsdale, E.; Grieco, C.; Kohler, B.; Bentley, W.E.; Shi, X.; Payne, G.F. Catechol-Based Molecular Memory Film for Redox Linked Bioelectronics. Adv. Electron. Mater. 2020, 6, 2000452. [Google Scholar] [CrossRef]
- Kim, E.; Xiong, Y.; Cheng, Y.; Wu, H.-C.; Liu, Y.; Morrow, B.H.; Ben-Yoav, H.; Ghodssi, R.; Rubloff, G.W.; Shen, J.; et al. Chitosan to Connect Biology to Electronics: Fabricating the Bio-Device Interface and Communicating Across This Interface. Polymers 2015, 7, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.; Kim, E.; Liu, Y.; Kang, M.; Li, J.; Yin, J.-J.; Liu, H.; Qu, X.; Liu, C.; Bentley, W.E.; et al. Radical Scavenging Activities of Biomimetic Catechol-Chitosan Films. Biomacromolecules 2018, 19, 3502–3514. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Liu, H.; Zhang, C.; Lei, Y.; Lei, M.; Xu, M.; Jin, D.; Li, P.; Yin, M.; Payne, G.F.; et al. Electrofabrication of functional materials: Chloramine-based antimicrobial film for infectious wound treatment. Acta Biomater. 2018, 73, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Truc, N.T.; Minh, H.H.; Khanh, L.L.; Thuy, V.M.; Van Toi, V.; Van Man, T.; Nam, H.C.N.; Quyen, T.N.; Hiep, N.T. Modification of type I collagen on TiO2 surface using electrochemical deposition. Surf. Coat. Technol. 2018, 344, 664–672. [Google Scholar] [CrossRef]
- Hu, P.; Rooholghodos, S.A.; Pham, L.H.; Ly, K.L.; Luo, X. Interfacial Electrofabrication of Freestanding Biopolymer Membranes with Distal Electrodes. Langmuir 2020, 36, 11034–11043. [Google Scholar] [CrossRef]
- Li, J.; Wu, S.; Kim, E.; Yan, K.; Liu, H.; Liu, C.; Dong, H.; Qu, X.; Shi, X.; Shen, J.; et al. Electrobiofabrication: Electrically based fabrication with biologically derived materials. Biofabrication 2019, 11, 032002. [Google Scholar] [CrossRef]
- Lei, M.; Qu, X.; Liu, H.; Liu, Y.; Wang, S.; Wu, S.; Bentley, W.E.; Payne, G.F.; Liu, C. Programmable Electrofabrication of Porous Janus Films with Tunable Janus Balance for Anisotropic Cell Guidance and Tissue Regeneration. Adv. Funct. Mater. 2019, 29, 1900065. [Google Scholar] [CrossRef]
- Avcu, E.; Baştan, F.E.; Abdullah, H.Z.; Rehman, M.A.U.; Avcu, Y.Y.; Boccaccini, A.R. Electrophoretic deposition of chitosan-based composite coatings for biomedical applications: A review. Prog. Mater. Sci. 2019, 103, 69–108. [Google Scholar] [CrossRef]
- Yan, K.; Liu, Y.; Zhang, J.; Correa, S.O.; Shang, W.; Tsai, C.-C.; Bentley, W.E.; Shen, J.; Scarcelli, G.; Raub, C.B.; et al. Electrical Programming of Soft Matter: Using Temporally Varying Electrical Inputs To Spatially Control Self Assembly. Biomacromolecules 2018, 19, 364–373. [Google Scholar] [CrossRef]
- Guo, X.; Huang, W.; Tong, J.; Chen, L.; Shi, X. One-step programmable electrofabrication of chitosan asymmetric hydrogels with 3D shape deformation. Carbohydr. Polym. 2022, 277, 118888. [Google Scholar] [CrossRef]
- Li, K.; Correa, S.O.; Pham, P.; Raub, C.B.; Luo, X. Birefringence of flow-assembled chitosan membranes in microfluidics. Biofabrication 2017, 9, 034101. [Google Scholar] [CrossRef] [PubMed]
- Ly, K.; Luo, X.; Raub, C.B. Oral mucositis on a chip: Modeling induction by chemo- and radiation treatments and recovery. Biofabrication 2022, 15, 015007. [Google Scholar] [CrossRef] [PubMed]
- Ly, K.L.; Rooholghodos, S.A.; Rahimi, C.; Rahimi, B.; Bienek, D.R.; Kaufman, G.; Raub, C.B.; Luo, X. An Oral-mucosa-on-a-chip sensitively evaluates cell responses to dental monomers. Biomed. Microdevices 2021, 23, 7. [Google Scholar] [CrossRef] [PubMed]
- Lam, V.K.; Phan, T.; Ly, K.; Luo, X.; Nehmetallah, G.; Raub, C.B. Dual-modality digital holographic and polarization microscope to quantify phase and birefringence signals in biospecimens with a complex microstructure. Biomed. Opt. Express 2022, 13, 805–823. [Google Scholar] [CrossRef]
- Ly, K.L.; Hu, P.; Pham, L.H.; Lam, V.; Raub, C.B.; Luo, X. Electrofabricated chitosan membrane arrays and their physicochemical properties in microfluidics. In Proceedings of the 25th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Palm Springs, CA, USA, 10–14 October 2021. [Google Scholar]
- Pham, P.; Vo, T.; Luo, X. Steering air bubbles with an add-on vacuum layer for biopolymer membrane biofabrication in PDMS microfluidics. Lab Chip 2017, 17, 248–255. [Google Scholar] [CrossRef]
- Luo, X.; Wu, H.-C.; Betz, J.; Rubloff, G.W.; Bentley, W.E. Air bubble-initiated biofabrication of freestanding, semi-permeable biopolymer membranes in PDMS microfluidics. Biochem. Eng. J. 2014, 89, 2–9. [Google Scholar] [CrossRef]
- Ly, K.L.; Luo, X. Fabrication and Characterization of Porous Flow-Assembled Chitosan Membranes in Microfluidics. In Proceedings of 8th International Conference on the Development of Biomedical Engineering in Vietnam; Springer Nature: Cham, Switzerland, 2022; pp. 383–392. [Google Scholar]
- Ly, K.L.; Raub, C.B.; Luo, X. Tuning the porosity of biofabricated chitosan membranes in microfluidics with co-assembled nanoparticles as templates. Mater. Adv. 2020, 1, 34–44. [Google Scholar] [CrossRef]
- Kocsis, K.; Hyttinen, M.; Helminen, H.J.; Aydelotte, M.B.; Módis, L. Combination of digital image analysis and polarization microscopy: Theoretical considerations and experimental data. Microsc. Res. Tech. 1998, 43, 511–517. [Google Scholar] [CrossRef]
- Hu, P.; Raub, C.B.; Choy, J.S.; Luo, X. Modulating the properties of flow-assembled chitosan membranes in microfluidics with glutaraldehyde crosslinking. J. Mater. Chem. B 2020, 8, 2519–2529. [Google Scholar] [CrossRef]
- Raub, C.B.; Hsu, S.C.; Chan, E.F.; Shirazi, R.; Chen, A.C.; Chnari, E.; Semler, E.J.; Sah, R.L. Microstructural remodeling of articular cartilage following defect repair by osteochondral autograft transfer. Osteoarthr. Cartil. 2013, 21, 860–868. [Google Scholar] [CrossRef] [Green Version]
- Rieppo, J.; Hallikainen, J.; Jurvelin, J.S.; Kiviranta, I.; Helminen, H.J.; Hyttinen, M.M. Practical considerations in the use of polarized light microscopy in the analysis of the collagen network in articular cartilage. Microsc. Res. Tech. 2008, 71, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Berlin, D.L.; Betz, J.; Payne, G.F.; Bentley, W.E.; Rubloff, G.W. In situ generation of pH gradients in microfluidic devices for biofabrication of freestanding, semi-permeable chitosan membranes. Lab Chip 2010, 10, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Liang, C.; Sun, S.; He, L.; Gao, D. On-Chip Fabrication of Carbon Nanoparticle–Chitosan Composite Membrane. J. Mater. Sci. Technol. 2015, 31, 1087–1093. [Google Scholar] [CrossRef]
- Cross, E.R. The electrochemical fabrication of hydrogels: A short review. SN Appl. Sci. 2020, 2, 397. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.-Q.; Lee, K.; Wang, X.; English, D.S.; Losert, W.; Payne, G.F. Chitosan-Mediated and Spatially Selective Electrodeposition of Nanoscale Particles. Langmuir 2005, 21, 3641–3646. [Google Scholar] [CrossRef]
- Fernandes, R.; Wu, L.-Q.; Chen, T.; Yi, H.; Rubloff, G.W.; Ghodssi, R.; Bentley, W.E.; Payne, G.F. Electrochemically Induced Deposition of a Polysaccharide Hydrogel onto a Patterned Surface. Langmuir 2003, 19, 4058–4062. [Google Scholar] [CrossRef]
- Wu, S.; Yan, K.; Li, J.; Huynh, R.N.; Raub, C.B.; Shen, J.; Shi, X.; Payne, G.F. Electrical cuing of chitosan’s mesoscale organization. React. Funct. Polym. 2020, 148, 104492. [Google Scholar] [CrossRef]
- Nagy, K.; Sipos, O.; Valkai, S.; Gombai, E.; Hodula, O.; Kerenyi, A.; Ormos, P.; Galajda, P. Microfluidic study of the chemotactic response of Escherichia coli to amino acids, signaling molecules and secondary metabolites. Biomicrofluidics 2015, 9, 044105. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Pinelis, M.; Maharbiz, M.M. Generating steep, shear-free gradients of small molecules for cell culture. Biomed. Microdevices 2009, 11, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.M.; Duarte, A.R.C.; Caridade, S.G.; Picart, C.; Reis, R.L.; Mano, J.F. Tailored Freestanding Multilayered Membranes Based on Chitosan and Alginate. Biomacromolecules 2014, 15, 3817–3826. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Wang, Z.; Liu, W.; Imai, M. Preparation of free-standing chitosan membranes for Chlorella vulgaris harvest and examination on suppression of algae-fouling properties. Water Environ. J. 2019, 33, 300–309. [Google Scholar] [CrossRef]
- Caridade, S.G.; Monge, C.; Gilde, F.; Boudou, T.; Mano, J.F.; Picart, C. Free-Standing Polyelectrolyte Membranes Made of Chitosan and Alginate. Biomacromolecules 2013, 14, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-Q.; Gadre, A.P.; Yi, H.; Kastantin, M.J.; Rubloff, G.W.; Bentley, W.E.; Payne, G.F.; Ghodssi, R. Voltage-Dependent Assembly of the Polysaccharide Chitosan onto an Electrode Surface. Langmuir 2002, 18, 8620–8625. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Gray, K.M.; Cheng, Y.; Kim, E.; Rubloff, G.W.; Bentley, W.E.; Wang, Q.; Payne, G.F. Electrodeposition of a weak polyelectrolyte hydrogel: Remarkable effects of salt on kinetics, structure and properties. Soft Matter 2013, 9, 2703–2710. [Google Scholar] [CrossRef]
- Feldman, D. The theory of polymer dynamics, by M. Doi and S. F. Edwards, the Clarendon Press, Oxford University Press, New York, 1986, 391 pp. Price: $78.50. J. Polym. Sci. Polym. Lett. Ed. 1989, 27, 239–240. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, Y.; Xiao, L.; Deng, H.; Du, Y.; Shi, X. Electrochemical deposition to construct a nature inspired multilayer chitosan/layered double hydroxides hybrid gel for stimuli responsive release of protein. J. Mater. Chem. B 2015, 3, 7577–7584. [Google Scholar] [CrossRef]
- Jia, N.; Rosella, E.; Juère, E.; Pouliot, R.; Kleitz, F.; Greener, J. A microfluidic approach to micromembrane synthesis for complex release profiles of nanocarriers. Lab Chip 2020, 20, 1066–1071. [Google Scholar] [CrossRef]
- Koev, S.T.; Dykstra, P.H.; Luo, X.; Rubloff, G.W.; Bentley, W.E.; Payne, G.F.; Ghodssi, R. Chitosan: An integrative biomaterial for lab-on-a-chip devices. Lab Chip 2010, 10, 3026–3042. [Google Scholar] [CrossRef]








| Sample | pH of Chitosan | pH of Alginate | Voltage (V) |
|---|---|---|---|
| pH6_3V | 5.5 | 6 | 3 |
| pH6_4.5V | 5.5 | 6 | 4.5 |
| pH6_6V | 5.5 | 6 | 6 |
| pH8_3V | 5.5 | 8 | 3 |
| pH8_4.5V | 5.5 | 8 | 4.5 |
| pH8_6V | 5.5 | 8 | 6 |
| pH10_3V | 5.5 | 10 | 3 |
| pH10_4.5V | 5.5 | 10 | 4.5 |
| pH10_6V | 5.5 | 10 | 6 |
| pH11.5_3V | 5.5 | 11.5 | 3 |
| pH11.5_4.5V | 5.5 | 11.5 | 4.5 |
| pH11.5_6V | 5.5 | 11.5 | 6 |
| pH11.5_flow | 5.5 | 11.5 | 0 |
| Multiple Linear Regression Parameter | Thickness | Semi-Permeability | Retardance |
|---|---|---|---|
| Constant | 0.07 ± 0.07 | 1.83 ± 0.11 | −0.07 ± 0.11 |
| Regression coefficient of pH (unit: 1/unit pH) | 0.03 ± 0.01 | −0.04 ± 0.01 | 0.04 ± 0.01 |
| Regression coefficient of voltage (unit: 1/volts) | 0.1 ± 0.01 | −0.23 ± 0.02 | 0.11 ± 0.02 |
| Multiple R2 | 0.959 | 0.981 | 0.935 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ly, K.L.; Hu, P.; Raub, C.B.; Luo, X. Programmable Physical Properties of Freestanding Chitosan Membranes Electrofabricated in Microfluidics. Membranes 2023, 13, 294. https://doi.org/10.3390/membranes13030294
Ly KL, Hu P, Raub CB, Luo X. Programmable Physical Properties of Freestanding Chitosan Membranes Electrofabricated in Microfluidics. Membranes. 2023; 13(3):294. https://doi.org/10.3390/membranes13030294
Chicago/Turabian StyleLy, Khanh L., Piao Hu, Christopher B. Raub, and Xiaolong Luo. 2023. "Programmable Physical Properties of Freestanding Chitosan Membranes Electrofabricated in Microfluidics" Membranes 13, no. 3: 294. https://doi.org/10.3390/membranes13030294
APA StyleLy, K. L., Hu, P., Raub, C. B., & Luo, X. (2023). Programmable Physical Properties of Freestanding Chitosan Membranes Electrofabricated in Microfluidics. Membranes, 13(3), 294. https://doi.org/10.3390/membranes13030294