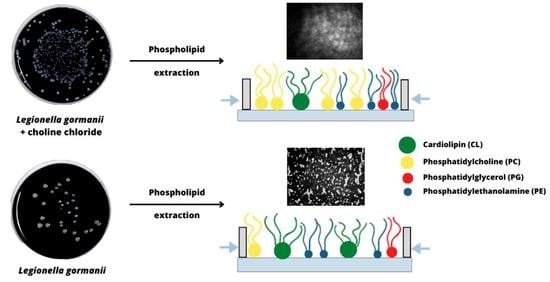
Physicochemical Characteristics of Model Membranes Composed of Legionella gormanii Lipids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Media
2.2. Extraction and Separation of Phospholipid by TLC Plates
2.3. Preparation of Fatty Acid Methyl Esters (FAMEs)
2.4. Gas-Liquid Chromatography and Mass Spectrometry
2.5. Langmuir Monolayer Studies
2.6. Statistical Analysis
3. Results
3.1. Fatty Acid Composition of Phospholipids
3.2. Isotherms and Compression Modulus
3.3. Surface Potential
3.4. Morphology
4. Discussion
5. Conclusions
- External conditions, such as temperature or presence of choline, have an impact on the bacterial membrane composition and in consequence, the physicochemical properties of the cell.
- The obtained results exhibited differences in the content of fatty acids present in individual phospholipids extracted from bacteria cultured on a medium with and without choline. These differences made it possible to explain the changes in the degree of packing and ordering of monolayers.
- The physical state of phospholipid monolayers can determine the interactions with external factors acting on bacteria. The denser packing and ordering of L. gormanii membranes composed of PLs extracted from bacteria grown on the medium with choline can suggest that such bacteria will be more resistant to the bactericidal action of agents inside the nutrient-rich but hostile environment of the host.
- Domain formation of different condensation and composition can be of great importance for L. gormanii membrane functioning, including proper activity of proteins, and developing the interaction mechanisms with the host cell and antibacterial substances.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Ephros, M.; Engelhard, D.; Maayan, S.; Bercovier, H.; Avital, A.; Yatsiv, I. L. gormanii pneumonia in a child with chronic granulomatous disease. Pediatr. Infect. Dis. J. 1989, 8, 726–727. [Google Scholar] [PubMed]
- Lei, C.; Zhou, X.; Ding, S.; Xu, Y.; Yang, B.; Guo, W.; Song, M.; Yang, M.; Jia, Y.; Luo, H. Case Report: Community-acquired Legionella gormanii pneumonia in an immunocompetent patient detected by metagenomic next-generation sequencing. Front. Med. 2022, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.; Chiou, C.C.; Famigilleti, R.; Lee, T.C.; Yu, V.L. Problem pathogens: Paediatric legionellosis—implications for improved diagnosis. Lancet Infect. Dis. 2006, 6, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.; Rydzewski, K.; Broich, M.; Schunder, E.; Heuner, K.; Flieger, A. Phospholipase PlaB of Legionella pneumophila represents a novel lipase family: Protein residues essential for lipolytic activity, substrate specificity, and hemolysis. J. Biol. Chem. 2009, 284, 27185–27194. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Valero, L.; Rusniok, C.; Carson, D.; Mondino, S.; Pérez-Cobas, A.E.; Rolando, M.; Pasrichad, S.; Reutere, S.; Demirtas, J.; Crumbach, J.; et al. More than 18,000 effectors in the Legionella genus genome provide multiple, independent combinations for replication in human cells. Proc. Natl. Acad. Sci. USA 2019, 116, 2265–2273. [Google Scholar] [CrossRef] [Green Version]
- Qin, T.; Zhou, H.; Ren, H.; Liu, W. Distribution of secretion systems in the genus Legionella and its correlation with pathogenicity. Front. Microbiol. 2017, 8, 388. [Google Scholar] [CrossRef] [Green Version]
- Corradi, V.; Sejdiu, B.I.; Mesa-Galloso, H.; Abdizadeh, H.; Noskov, S.Y.; Marrink, S.J.; Tieleman, D.P. Emerging diversity in lipid-protein interactions. Chem. Rev. 2019, 119, 5775–5848. [Google Scholar] [CrossRef] [Green Version]
- Palusinska-Szysz, M.; Szuster-Ciesielska, A.; Janczarek, M.; Wdowiak-Wróbel, S.; Schiller, J.; Reszczynska, E.; Gruszecki, W.I.; Fuchs, B. Genetic diversity of Legionella pcs and pmtA genes and the effect of utilization of choline by Legionella spp. on induction of proinflammatory cytokines. Pathog. Dis. 2019, 77, ftz065. [Google Scholar] [CrossRef]
- Chmiel, E.; Galuska, C.E.; Koper, P.; Kowalczyk, B.; Urbanik-Sypniewska, T.; Palusińska-Szysz, M.; Fuchs, B. Unusual lipid components of Legionella gormanii membranes. Metabolites 2022, 12, 418. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, J.W. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Amemura-Maekawa, J.; Hayakawa, Y.; Sugie, H.; Moribayashi, A.; Kura, F.; Chang, B.; Wada, A.; Watanabe, H. Legioliulin, a new isocoumarin compound responsible for blue-white autofluorescence in Legionella (Fluoribacter) dumoffi under long-wavelength UV light. Biochem. Biophys. Res. Commun. 2004, 323, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.T.; Rideal, E.K. Interfacial Phenomena; Academic Press: New York, NY, USA, 1963. [Google Scholar]
- Lee, K.Y.C. Collapse mechanisms of Langmuir monolayers. Annu. Rev. Phys. Chem. 2008, 59, 771–791. [Google Scholar] [CrossRef] [Green Version]
- Kaganer, V.M.; Möhwald, H.; Dutta, P. Structure and phase transitions in Langmuir monolayers. Rev. Mod. Phys. 1999, 71, 779–819. [Google Scholar] [CrossRef] [Green Version]
- Ohshima, H.; Ohki, S. Donnan potential and surface potential of a charged membrane. Biophys. J. 1985, 47, 673–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, V.; Möbius, D. Local surface potentials and electric dipole moments of lipid monolayers: Contributions of the water/lipid and the lipid/air interfaces. J. Colloid Interface Sci. 1988, 126, 408–420. [Google Scholar] [CrossRef]
- Chachaj-Brekiesz, A.; Kobierski, J.; Wnętrzak, A.; Dynarowicz-Latka, P. Electrical properties of membrane phospholipids in Langmuir monolayers. Membranes 2021, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Chachaj-Brekiesz, A.; Kobierski, J.; Echaniz, R.G.; Wnętrzak, A.; Dynarowicz-Latka, P. Comprehensive approach to the interpretation of the electrical properties of film-forming molecules. J. Phys. Chem. B 2022, 126, 7037–7046. [Google Scholar] [CrossRef]
- Ridgway, N.D. Phospholipid synthesis in mammalian cells. In Biochemistry of Lipids, Lipoproteins and Membranes, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 209–236. [Google Scholar] [CrossRef]
- Caillon, L.; Nieto, V.; Gehan, P.; Omrane, M.; Rodriguez, N.; Monticelli, L.; Thiam, A.R. Triacylglycerols sequester monotopic membrane proteins to lipid droplets. Nat. Commun. 2020, 11, 3944. [Google Scholar] [CrossRef]
- Murzyn, K.; Róg, T.; Pasenkiewicz-Gierula, M. Phosphatidylethanolamine-phosphatidylglycerol bilayer as a model of the inner bacterial membrane. Biophys. J. 2005, 88, 1091–1103. [Google Scholar] [CrossRef] [Green Version]
- Phan, M.D.; Shin, K. Effects of cardiolipin on membrane morphology: A Langmuir monolayer study. Biophys. J. 2015, 108, 1977–1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Róg, T.; Gurtovenko, A.A.; Vattulainen, I.; Karttunen, M. Role of phosphatidylglycerols in the stability of bacterial membranes. Biochimie 2008, 90, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.O.; Schulman, J.H. The ionic structure of lecithin monolayers. J. Lipid Res. 1967, 8, 227–233. [Google Scholar] [CrossRef]
- Leekumjorn, S.; Sum, A.K. Molecular simulation study of structural and dynamic properties of mixed DPPC/DPPE bilayers. Biophys. J. 2006, 90, 3951–3965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möhwald, H. Phospholipid and phospholipid-protein monolayers at the air/water interface. Annu. Rev. Phys. Chem. 1990, 41, 441–476. [Google Scholar] [CrossRef]
- Ma, G.; Allen, H.C. DPPC Langmuir monolayer at the air-water interface: Probing the tail and headgroups by vibrational sum frequency generation spectroscopy. Langmuir 2006, 22, 5341–5349. [Google Scholar] [CrossRef]
- Wydro, P. The influence of cardiolipin on phosphatidylglycerol/phosphatidylethanolamine monolayers—Studies on ternary films imitating bacterial membranes. Colloids Surf. B Biointerfaces 2013, 106, 217–223. [Google Scholar] [CrossRef]
- Epand, R.M.; Epand, R.F. Domains in bacterial membranes and the action of antimicrobial agents. Mol. Biosyst. 2009, 5, 580–587. [Google Scholar] [CrossRef]
- Vanounou, S.; Parola, A.H.; Fishov, I. Phosphatidylethanolamine and phosphatidylglycerol are segregated into different domains in bacterial membrane. A study with pyrene-labelled phospholipids. Mol. Microbiol. 2003, 49, 1067–1079. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kusaka, J.; Nishibori, A.; Hara, H. Lipid domains in bacterial membranes. Mol. Microbiol. 2006, 61, 1110–1117. [Google Scholar] [CrossRef]
- Garcia, A.; Zou, H.; Hossain, K.R.; Xu, Q.H.; Buda, A.; Clarke, R.J. Polar interactions play an important role in the energetics of the main phase transition of phosphatidylcholine membranes. ACS Omega 2019, 4, 518–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brockman, H. Dipole potential of lipid membranes. Chem. Phys. Lipids 1994, 37, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Jurak, M.; Szafran, K.; Cea, P.; Martín, S. Analysis of molecular interactions between components in phospholipid-immunosuppressant-antioxidant mixed Langmuir films. Langmuir 2021, 37, 5601–5616. [Google Scholar] [CrossRef]
- Oliveira, O.N., Jr.; Bonardi, C. The surface potential of Langmuir monolayers revisited. Langmuir 1997, 13, 5920–5924. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, O.N., Jr.; Riul, A., Jr.; Leite, V.B.P. Water at interfaces and its influence on the electrical properties of adsorbed films. Braz. J. Phys. 2004, 34, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Klaiss-Luna, M.C.; Manrique-Moreno, M. Infrared spectroscopic study of multi-component lipid systems: A closer approximation to biological membrane fluidity. Membranes 2022, 12, 534. [Google Scholar] [CrossRef]
- Palusińska-Szysz, M.; Jurak, M.; Gisch, N.; Waldow, F.; Zehethofer, N.; Nehls, C.; Schwudke, D.; Koper, P.; Mazur, A. The human LL-37 peptide exerts antimicrobial activity against Legionella micdadei interacting with membrane phospholipids. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159138. [Google Scholar] [CrossRef] [PubMed]
- Dannehl, C.; Brezesinski, G.; Möhwald, H. Interactions of two fragments of the human antimicrobial peptide LL-37 with zwitteronic and anionic lipid monolayers. Z. Phys. Chem. 2015, 229, 1141–1159. [Google Scholar] [CrossRef] [Green Version]






| Phospholipid Class | Molecular Weight (g/mol) |
| PC−choline | 730.6 |
| PC+choline | 729.4 |
| PE−choline | 683.5 |
| PE+choline | 680.9 |
| CL−choline | 1389.7 |
| CL+choline | 1385.9 |
| PG−choline | 715.8 |
| PG+choline | 714.0 |
| Phospholipid Mixture | Molecular Weight (g/mol) |
| PL−choline | 845.0 |
| PL+choline | 789.3 |
| Sum of Saturated FA | Sum of Unsaturated FA | Sum of FA 14–18 | Sum of FA 19–21 | Percentage of Phospholipid Class in PL Mixture [9] | |
|---|---|---|---|---|---|
| PC−choline | 77.0 ± 0.6 | 23.0 ± 0.5 | 97.5 ± 0.7 | 2.5 ± 0.3 | 26.0 ± 2.0 |
| PC+choline | 75.0 ± 0.5 | 24.0 ± 0.4 | 94.0 ± 0.5 * | 6.0 ± 0.3 * | 47.0 ± 0.0 |
| PE−choline | 79.0 ± 0.7 | 21.0 ± 0.2 | 96.0 ± 0.7 | 4.0 ± 0.1 | 50.0 ± 1.4 |
| PE+choline | 79.5 ± 0.3 | 20.5 ± 0.3 | 95.5 ± 0.4 | 4.5 ± 0.2 | 38.0 ± 0.6 |
| CL−choline | 76.0 ± 0.5 | 24.0 ± 0.8 | 88.0 ± 0.6 | 12.0 ± 0.8 | 21.0 ± 1.4 |
| CL+choline | 75.0 ± 0.4 | 25.0 ± 0.6 | 91.0 ± 0.5 * | 9.0 ± 0.6 * | 12.0 ± 0.6 |
| PG−choline | 89.0 ± 0.9 | 11.0 ± 1.0 | 83.0 ± 1.0 | 17.0 ± 0.6 | 3.0 ± 0.7 |
| PG+choline | 87.0 ± 1.0 | 13.0 ± 1.0 | 89.0 ± 1.0 * | 11.0 ± 0.7 * | 3.0 ± 0.6 |
| Monolayer | (mN/m) | (mN/m) | (mN/m) | ||
|---|---|---|---|---|---|
| PL−choline (20 °C) | 99 | 49 | 108 | 39 | 49 |
| PL−choline (37 °C) | 108 | 46 | 109 | 38 | 54 |
| PL+choline (20 °C) | 71 | 48 | 119 | 37 | 39 |
| PL+choline (37 °C) | 76 | 46 | 116 | 33 | 44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastuszak, K.; Chmiel, E.; Kowalczyk, B.; Tarasiuk, J.; Jurak, M.; Palusińska-Szysz, M. Physicochemical Characteristics of Model Membranes Composed of Legionella gormanii Lipids. Membranes 2023, 13, 356. https://doi.org/10.3390/membranes13030356
Pastuszak K, Chmiel E, Kowalczyk B, Tarasiuk J, Jurak M, Palusińska-Szysz M. Physicochemical Characteristics of Model Membranes Composed of Legionella gormanii Lipids. Membranes. 2023; 13(3):356. https://doi.org/10.3390/membranes13030356
Chicago/Turabian StylePastuszak, Katarzyna, Elżbieta Chmiel, Bożena Kowalczyk, Jacek Tarasiuk, Małgorzata Jurak, and Marta Palusińska-Szysz. 2023. "Physicochemical Characteristics of Model Membranes Composed of Legionella gormanii Lipids" Membranes 13, no. 3: 356. https://doi.org/10.3390/membranes13030356
APA StylePastuszak, K., Chmiel, E., Kowalczyk, B., Tarasiuk, J., Jurak, M., & Palusińska-Szysz, M. (2023). Physicochemical Characteristics of Model Membranes Composed of Legionella gormanii Lipids. Membranes, 13(3), 356. https://doi.org/10.3390/membranes13030356