Green Fabrication of Sustainable Porous Chitosan/Kaolin Composite Membranes Using Polyethylene Glycol as a Porogen: Membrane Morphology and Properties
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
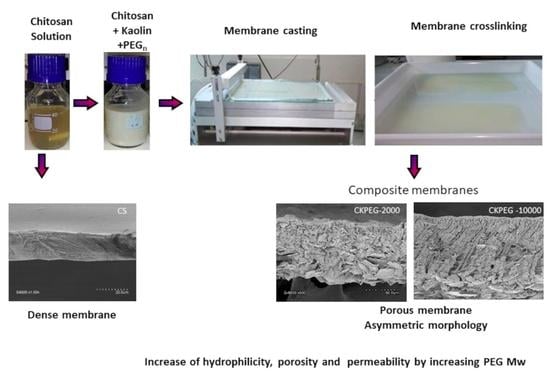
2.2. Composite Membrane Preparation
2.3. Rheological Properties of Casting Mixtures
2.4. Membrane Characterization
2.4.1. Membrane Morphology and Pore Size Determination
2.4.2. Mechanical Property Measurements
2.4.3. Thermal Stability
2.4.4. Water Solubility
2.4.5. Water Contact Angle (WCA) Measurement
2.4.6. Infrared Spectra Measurements
2.4.7. Filtration Tests
3. Results and Discussion
3.1. Rheological Properties of Casting Mixtures
3.2. Membrane Morphology
3.3. Surface Porosity, Average Pore Size and Pore Size Distribution
3.4. Surface Properties of CS/KO Membranes
3.5. Physical Properties of the Composite Membranes
3.6. Membrane Permeability
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowska, M.A. The Use of Polymer Inclusion Membranes for the Removal of Metal Ions from Aqueous Solutions—The Latest Achievements and Potential Industrial Applications: A Review. Membranes 2022, 12, 1135. [Google Scholar] [CrossRef] [PubMed]
- Saja, S.; Bouazizi, A.; Achiou, B.; Ouaddari, H.; Karim, A.; Ouammou, M.; Aaddane, A.; Bennazha, J.; Alami Younssi, S. Fabrication of low-cost ceramic ultrafiltration membrane made from bentonite clay and its application for soluble dyes removal. J. Eur. Ceram. Soc. 2020, 40, 2453–2462. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellon, C.; Loutatidou, S.; Karimi, L.; Mikeloni, A.M.; Achilli, A.; Ghassemi, A.; et al. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2018, 81, 209–237. [Google Scholar] [CrossRef]
- Alaa, M.; Samy, Y. Green and sustainable membrane fabrication development. Sustain. Technol. Green Econ. 2021, 1, 14–23. [Google Scholar]
- Adeniyi, A.; Gonzalez-Ortiz, D.; Pochat-Bohatier, C.; Oyewo, O.; Sithole, B.; Onyango, M. Incorporation of Cellulose Nanocrystals (CNC) derived from sawdust into polyamide thin-film composite membranes for enhanced water recovery. Alex. Eng. J. 2020, 59, 4201–4210. [Google Scholar] [CrossRef]
- Hanafia, A.; Faur, C.; Deratani, A.; Guenoun, P.; Garate, H.; Quemener, D.; Pochat-Bohatier, C.; Bouyer, B. Fabrication of novel porous membrane from biobased water-soluble polymer (hydroxypropylcellulose). J. Membr. Sci. 2017, 526, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Kamal, O.; Pochat-Bohatier, C.; Marcano, J.S.C. Development and stability of gelatin cross-linked membranes for copper (II) ions removal from acid waters. Sep. Purif. Technol. 2017, 183, 153–161. [Google Scholar] [CrossRef]
- Vatanpour, V.; Yavuzturk Gul, B.; Zeytuncu, B.; Korkut, S.; İlyasoǧlu, G.; Turken, T.; Badawi, M.; Koyuncu, I.; Saeb, M.R. Polysaccharides in fabrication of membranes: A review. Carbohydr. Polym. 2022, 281, 119041. [Google Scholar] [CrossRef] [PubMed]
- Bassyouni, M.; Abdel-Aziz, M.H.; Zoromba, M.S.; Abdel-Hamid, S.M.S.; Drioli, E. A review of polymeric nanocomposite membranes for water purification. J. Ind. Eng. Chem. 2019, 73, 19–46. [Google Scholar] [CrossRef]
- Jaafar, J.; Nasir, A.M. Grand Challenge in Membrane Fabrication: Membrane Science and Technology. Front. Membr. Sci. Technol. 2022, 1, 883913. [Google Scholar] [CrossRef]
- Yadav, P.; Ismail, N.; Essalhi, M.; Tysklind, M.; Athanassiadis, D.; Tavajohi, N. Assessment of the Environmental Impact of Polymeric Membrane Production. J. Membr. Sci. 2021, 622, 118987. [Google Scholar] [CrossRef]
- Kim, D.; Nunes, S.P. Green Solvents for Membrane Manufacture: Recent Trends and Perspectives. Curr. Opin. Green Sustain. Chem. 2021, 28, 100427. [Google Scholar] [CrossRef]
- M’barki, O.; Hanafia, A.; Bouyer, D.; Faur, C.; Sescousse, R.; Delabre, U.; Blot, C.; Guenoun, P.; Deratani, A.; Quemener, D.; et al. Greener method to prepare porous polymer membranes by combining thermally induced phase separation and crosslinking of poly (vinyl alcohol) in water. J. Membr. Sci. 2014, 458, 225–235. [Google Scholar] [CrossRef]
- Prasanna, S.B.; Selvakumara, D.; Kadirveluab, K.; Kumara, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar]
- Silvestre, W.P.; Baldasso, C.; Tessaro, I.C. Potential of chitosan-based membranes for the separation of essential oil components by target-organophilic pervaporation. Carbohydr. Polym. 2020, 247, 116676. [Google Scholar] [CrossRef]
- Yu, Y.; Xie, C.; Wu, Y.; Liu, P.; Wan, Y.; Sun, X.; Wang, L.; Zhang, Y. Preparation of a PVA/Chitosan/Glass Fiber Composite Membrane and the Performance in CO2 Separation. Membranes 2023, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Uragami, T.; Saito, T.; Miyata, T. Pervaporative dehydration characteristics of an ethanol/ water azeotrope through various chitosan membranes. Carbohydr. Polym. 2015, 120, 1–6. [Google Scholar] [CrossRef]
- Bouzid Rekik, S.; Gassara, S.; Bouaziz, J.; Deratani, A.; Baklouti, S. Development and characterization of porous membranes based on kaolin/chitosan composite. Appl. Clay Sci. 2017, 168, 312–323. [Google Scholar]
- Bouzid Rekik, S.; Gassara, S.; Bouaziz, J.; Deratani, A.; Baklouti, S. Enhancing hydrophilicity and permeation flux of chitosan/kaolin composite membranes by using polyethylene glycol as porogen. Appl. Clay Sci. 2019, 168, 312–323. [Google Scholar] [CrossRef]
- Bouzid Rekik, S.; Gassara, S.; Bouaziz, J.; Baklouti, S.; Deratani, A. Performance Enhancement of Kaolin/Chitosan Composite-Based Membranes by Cross-Linking with Sodium Tripolyphosphate: Preparation and Characterization. Membranes 2023, 13, 229. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shi, F.; Ma, J.; Wu, M.; Zhang, J.; Gao, C. Effect of PEG additive on the morphology and performance of polysulfone ultrafiltration membranes. Desalination 2011, 272, 51–58. [Google Scholar] [CrossRef]
- Lin, Y.; Tseng, H.; Wang, D.K. Uncovering the effects of PEG porogen molecular weight and concentration on ultrafiltration membrane properties and protein purification performance. J. Membr. Sci. 2021, 618, 118729. [Google Scholar] [CrossRef]
- Ortiz, D.G.; Nouxet, M.; Maréchal, W.; Lorain, O.; Deratani, A.; Pochat-Bohatier, C. Immobilization of Poly(vinyl pyrrolidone) in Polysulfone Membranes by Radically-Initiated Crosslinking Using Potassium Persulfate. Membranes 2022, 12, 664. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Usosky, V.V.; Volkov, V.V. Influence of the concentration and molecular weight of polyethylene glycol on the structure and permeability of polysulfone hollow fiber membranes. Pet. Chem. 2016, 56, 321–329. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Karslyan, Y.; Ovcharova, A.; Volkov, V.V. Development of High Flux Ultrafiltration Polyphenylsulfone Membranes Applying the Systems with Upper and Lower Critical Solution Temperatures: Effect of Polyethylene Glycol Molecular Weight and Coagulation Bath Temperature. J. Membr. Sci. 2018, 565, 266–280. [Google Scholar] [CrossRef]
- Pochat, C.; Venault, A.; Bouyer, D.; Vachoud, L.; David, L.; Faur, C. Development and Characterization of Composite Chitosan/Active Carbon Hydrogels for a Medical Application. J. Appl. Polym. Sci. 2013, 128, 2945–2953. [Google Scholar]
- Chen, S.; Ma, Z.; Qiao, F.; Sun, Y.; Yang, X.; Deng, X.; Cen, L.; Cai, Q.; Wu, M.; Zhang, X.; et al. Guided bone regeneration with tripolyphosphate cross-linked asymmetric chitosan membrane. J. Dent. 2014, 42, 1603–1612. [Google Scholar]
- Gassara, S.; Chinpa, W.; Quemener, D.; Ben Amar, R.; Deratani, A. Pore size tailoring of poly(etherimide) membrane from UF to NF range by chemical post-treatment using aminated oligomers. J. Membr. Sci. 2013, 436, 36–46. [Google Scholar] [CrossRef]
- Oh, H.J.; Freeman, B.D.; McGrath, J.E.; Ellison, C.J.; Mecham, S.; Lee, K.; Paul, C. Rheological studies of disulfonated poly(arylene ether sulfone) plasticized with poly(ethylene glycol) for membrane formation. Polymer 2014, 55, 1574–1582. [Google Scholar] [CrossRef]
- Yap Ang, M.B.M.; Lau, V., Jr.; Ji, Y.-L.; Huang, S.-H.; An, Q.-F.; Caparanga, A.R.; Tsai, H.-A.; Hung, W.-S.; Hu, C.-C.; Lee, K.-R.; et al. Correlating PSf Support Physicochemical Properties with the Formation of Piperazine-Based Polyamide and Evaluating the Resultant Nanofiltration Membrane Performance. Polymers 2017, 9, 505. [Google Scholar] [CrossRef]
- Soros, A.; Amburgey, J.E.; Stauber, C.E.; Sobseyand, D.; Casanova, L.M. Turbidity reduction in drinking water by coagulation-flocculation with chitosan polymers. J. Water Health 2019, 17, 204–218. [Google Scholar] [CrossRef] [Green Version]
- Kang, X.; Xia, Z.; Chen, R.; Liu, P.; Yang, W. Effects of inorganic cations and organic polymers on the physicochemical properties and microfabrics of kaolinite suspensions. Appl. Clay Sci. 2019, 176, 38–48. [Google Scholar] [CrossRef]
- Van de Witte, P.; Dijkstra, P.J.; Van den Berg, J.W.A.; Feijen, J. Phase separation processes in polymer solutions in relation to membrane formation. J. Membr. Sci. 1996, 117, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Hopp-Hirschler, M.; Nieken, U. Modeling of pore formation in phase inversion processes: Model and numerical results. J. Membr. Sci. 2018, 564, 820–831. [Google Scholar] [CrossRef]
- Fansuri, H.; Masyitoh, A.D.; Nurherdiana, S.; Utomo, W.; Gunawan, T.; Widiastuti, N.; Dzarfan Othman, M.H.; Ismail, A.F.; Subaer, S. A dependence study Molecular weight of polyethylene glycol (PEG) ON La0.7Sr0.3Co0.2Fe0.8O3-Ɣ (LSCF 7328) hollow fiber membrane for oxygen permeation. J. King Saud Univ. Eng. Sci. 2023, 35, 200–2006. [Google Scholar]
- Zhang, M.; Li, X.H.; Gong, Y.D.; Zhao, N.M.; Zhang, X.F. Properties and biocompatibility of chitosan films modified by blending with PEG. Biomaterials 2022, 23, 2641–2648. [Google Scholar] [CrossRef]
- Wongchitphimon, S.; Wang, R.; Jiraratananon, R.; Shi, L.; Loh, C.H. Effect of polyethylene glycol (PEG) as an additive on the fabrication of polyvinylidene fluoride-co-hexafluropropylene (PVDF-HFP) asymmetric microporous hollow fiber membranes. J. Membr. Sci. 2011, 369, 329–338. [Google Scholar] [CrossRef]
- Rekik, S.B.; Bouaziz, J.; Deratani, A.; Baklouti, S. Development of an Asymmetric Ultrafiltration Membrane from Naturally Occurring Kaolin Clays: Application for the Cuttlefish Effluents Treatment. J. Membr. Sci. Technol. 2016, 6, 159. [Google Scholar] [CrossRef]
- Du, Q.; Zhou, P.; Pan, Y.; Qu, X.; Liu, L.; Yu, H.; Hou, J. Influence of hydrophobicity and roughness on the wetting and flow resistance of water droplets on solid surface: A many-body dissipative particle dynamics study. Chem. Eng. Sci. 2022, 249, 117327. [Google Scholar] [CrossRef]
- Pereira, E.D.; Cerruti, R.; Fernandes, E.; Peña, L.; Saez, V.; Pinto, J.C.; Ramón, J.A.; Oliveira, G.E.; Souza, F.G. Influence of PLGA and PLGA-PEG on the dissolution profile of oxaliplatin. Polímeros 2016, 26, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Sapna, R.; Sharma, R.; Kumar, D. Chitosan-based membranes for wastewater desalination and heavy metal detoxification. In Nanoscale Materials in Water Purification; Sapna, R., Sharma, D., Kumar, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 799–814. [Google Scholar]
- Steenkamp, G.C.; Neomagus, H.W.J.P.; Krieg, H.M.; Keizer, K. Centrifugal casting of ceramic membrane tubes and the coating with chitosan. Sep. Pur. Technol. 2001, 25, 407–413. [Google Scholar] [CrossRef]
- Jana, S.; Saikia, A.; Purkait, M.K.; Mohanty, K. Chitosan based ceramic ultrafiltration membrane: Preparation, characterization and application to remove Hg(II) and As(III) using polymer enhanced ultrafiltration. Chem. Eng. J. 2011, 170, 209–219. [Google Scholar] [CrossRef]
- Karim, Z.; Mathew, A.P.; Grahn, M.; Mouzon, J.; Oksman, K. Nanoporous membranes with cellulose nanocrystals as functional entity in chitosan: Removal of dyes from water. Carbohydr. Polym. 2014, 112, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Abolhassani, M.; Griggs, C.S.; Gurtowski, L.A.; Mattei-Sosa, J.A.; Nevins, M.; Medina, V.F.; Morgan, T.A.; Greenlee, L.F. Scalable Chitosan-Graphene Oxide Membranes: The Effect of GO Size on Properties and Cross-Flow Filtration Performance. ACS Omega 2017, 2, 8751–8759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoerunnisa, F.; Rahmah, W.; Seng Ooi, B.; Dwihermiati, E.; Nashrah, N.; Fatimah, S.; Gun Ko, Y.; Ng, E.-P. Chitosan/PEG/MWCNT/Iodine composite membrane with enhanced antibacterial properties for dye wastewater treatment. J. Environ. Chem. Eng. 2020, 8, 103686. [Google Scholar] [CrossRef]
- Liu, T.; Graham, N.; Yu, W. Evaluation of a novel composite chitosan–graphene oxide membrane for NOM removal during water treatment. J. Environ. Chem. Eng. 2021, 9, 105716. [Google Scholar] [CrossRef]
- Bat-Amgalan, M.; Miyamoto, N.; Kano, N.; Yunden, G.; Kim, H.-J. Preparation and Characterization of Low-Cost Ceramic Membrane Coated with Chitosan: Application to the Ultrafine Filtration of Cr(VI). Membranes 2022, 12, 835. [Google Scholar] [CrossRef]











| Membranes | Component Part (w/v%) in 0.1 M Acetic Acid | |||||
|---|---|---|---|---|---|---|
| CS | KO | PEG 400 | PEG 2000 | PEG 6000 | PEG 10000 | |
| CS | 4 | - | - | - | - | - |
| CK | 4 | 5 | - | - | - | - |
| CKPEG-400 | 4 | 5 | 1 | - | - | - |
| CKPEG-2000 | 4 | 5 | - | 1 | - | - |
| CKPEG-6000 | 4 | 5 | - | - | 1 | - |
| CKPEG-10000 | 4 | 5 | - | - | - | 1 |
| Membrane | Method | CKPEG-400 | CKPEG-2000 | CKPEG-6000 | CKPEG-10000 |
|---|---|---|---|---|---|
| Average surface pore diameter (nm) | SEM image analysis | 50 ± 6 | 59 ± 7 | 71 ± 7 | 71 ± 3 |
| Nitrogen sorption–desorption | - | 38 | 44 | 45 | |
| Surface porosity (%) | SEM image analysis | 5 ± 2 | 9 ± 4 | 18 ± 4 | 17 ± 3 |
| Pore volume (cm3·g−1) | Nitrogen sorption–desorption | - | 0.0031 | 0.0028 | 0.0026 |
| Sample Name | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| CK-PEG-400 | 2 ± 0.03 | 8.2 ± 0.5 |
| CK-PEG-2000 | 2.8 ± 0.1 | 6.0 ± 0.1 |
| CK-PEG-6000 | 3.5 ± 0.3 | 5.8 ± 0.1 |
| CK5-PEG-10000 | 4 ± 0.3 | 4 ± 0.1 |
| CS | CK | CKPEG-400 | CKPEG-2000 | CKPEG-6000 | CKPEG-10000 | |
|---|---|---|---|---|---|---|
| pH = 9 | Insoluble | Insoluble | Insoluble | Insoluble | Insoluble | Insoluble |
| pH = 6.2 | Partially soluble | Insoluble | Insoluble | Insoluble | Insoluble | Insoluble |
| pH = 4 | Soluble | Partially soluble | Insoluble | Insoluble | Insoluble | Insoluble |
| pH = 2 | Soluble | Soluble | Insoluble | Insoluble | Insoluble | Insoluble |
| Membrane Type | Preparation Method | Structure | Pore Size(nm) | PWP a(L·h−1·m−2·bar−1) | Reference |
|---|---|---|---|---|---|
| MF/UF | Coating | CS/α-Al2O3 | 115 | 11 | [43] |
| MF/UF | Coating | GA b crosslinked CS/clay ceramic support | 36181 | 44900 | [44] |
| UF | Blending | GA b crosslinked CS/CNC c | 13–10 | 6.4 | [45] |
| NF | Blending | CS/GO d | n.d. | 0.58–1.31 | [46] |
| MF | Blending | CS/PEG6000/MWCNT c/iodine | n.d. | 105 | [47] |
| NF | Layer by layer | CS/GO d on PVDF UF membrane | n.d. | 1–2.5 | [48] |
| UF | Coating | GA b crosslinked CS/KO | 16 | 11 | [49] |
| UF | Blending | STPP crosslinked CS/PEG10000/KO | 71 | 150 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rekik, S.B.; Gassara, S.; Deratani, A. Green Fabrication of Sustainable Porous Chitosan/Kaolin Composite Membranes Using Polyethylene Glycol as a Porogen: Membrane Morphology and Properties. Membranes 2023, 13, 378. https://doi.org/10.3390/membranes13040378
Rekik SB, Gassara S, Deratani A. Green Fabrication of Sustainable Porous Chitosan/Kaolin Composite Membranes Using Polyethylene Glycol as a Porogen: Membrane Morphology and Properties. Membranes. 2023; 13(4):378. https://doi.org/10.3390/membranes13040378
Chicago/Turabian StyleRekik, Sonia Bouzid, Sana Gassara, and André Deratani. 2023. "Green Fabrication of Sustainable Porous Chitosan/Kaolin Composite Membranes Using Polyethylene Glycol as a Porogen: Membrane Morphology and Properties" Membranes 13, no. 4: 378. https://doi.org/10.3390/membranes13040378
APA StyleRekik, S. B., Gassara, S., & Deratani, A. (2023). Green Fabrication of Sustainable Porous Chitosan/Kaolin Composite Membranes Using Polyethylene Glycol as a Porogen: Membrane Morphology and Properties. Membranes, 13(4), 378. https://doi.org/10.3390/membranes13040378