Ionizing Radiation for Preparation and Functionalization of Membranes and Their Biomedical and Environmental Applications
Abstract
:1. Introduction
2. Ionizing Radiation
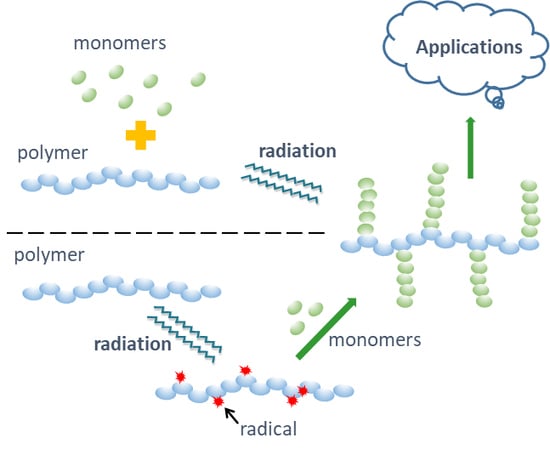
3. Membranes Preparation and/or Functionalization
4. Membranes Properties and Their Determination
5. Membranes Applications
5.1. Biomedical Applications
5.1.1. Controlled Drug Delivery
5.1.2. Tissue Regeneration
5.2. Environmental Applications
5.2.1. Energy Production
Fuel Cells
Proton-Exchange Membranes Fuel Cells (PEMFC)
Anion-Exchange Membranes Fuel Cells (AEMFC)
5.2.2. Energy Storage Devices
5.2.3. Water Treatment
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AEI | anion-exchange ionomer |
| AEM | anion-exchange membrane |
| AEMFC | anion-exchange membranes fuel cells |
| AFC | alkaline fuel cells |
| AIMFC | amphoteric ion-exchange membranes fuel cells |
| BTMA | benzyltrimethylammonium |
| CEM | cation-exchange membranes |
| DBL | diffusion boundary layer |
| DOG | degree of grafting |
| DMAEMA | dimethylaminoethyl methacrylate |
| DMFC | direct methanol fuel cells |
| EIS | electrochemical impedance spectroscopy |
| ETFE | poly(ethylene-co-tetrafluoroethylene) |
| FCD | fixed charge density |
| FEP | fluorinated ethylene propylene |
| GD | grafting degree |
| GY | grafting yield |
| HEMA | 2-hydroxyethyl methacrylate |
| HDPE | high-density polyethylene |
| HOR | hydrogen oxidation reaction |
| IEC | ion-exchange capacity |
| IEM | ion-exchange membrane |
| LDPE | low-density polyethylene |
| MEA | membrane electrode assembly |
| MCFC | molten carbonate fuel cells |
| MPRD | benzyl-N-methylpiperidinium |
| MPY | benzyl-N-methylpyrrolidinium |
| ORR | oxygen reduction reaction |
| PAFC | phosphoric acid fuel cells |
| PE | polyethylene |
| PEM | polymer electrolyte membrane |
| PEMFC | proton-exchange membranes fuel cells |
| PFSA | perfluorinated sulfonic acid |
| PHEMA | poly(hydroxyethyl methacrylate) |
| PP | polypropylene |
| PVBC | poly(vinylbenzyl chloride) |
| PVDF | polyvinylidene difluoride |
| PSSA | poly(styrene sulfonic acid) |
| QA | quaternary ammonium |
| RFB | redox flow batteries |
| RG | radiation-grafted |
| SD | swelling degree |
| SGE | salinity gradient energy |
| SGP | salinity gradient power |
| SHIs | swift heavy ions |
| SOFC | solid oxide fuel cells |
| SSS | sodium styrene sulfonate |
| St | styrene |
| TMA | benzyltrimethylammonium |
| VBC | vinylbenzyl chloride |
| VRFB | vanadium redox flow battery |
References
- Guven, O. Ionizing radiation: A verstile tool for nanostructuring of polymers. Pure Appl. Chem. 2016, 88, 1049–1061. [Google Scholar] [CrossRef]
- Rosiak, J.M. Radiation polymerization in solution. In IAEA-Tec-Doc-1420 Advances in Radiation Chemistry of Polymers; International Atomic Energy Agency: Vienna, Austria, 2004; pp. 41–60. [Google Scholar]
- Casimiro, M.H.; Botelho, M.L.; Leal, J.P.; Gil, M.H. Study on chemical, UV and gamma radiation-induced grafting of 2-hydroxyethyl methacrylate onto chitosan. Radiat. Phys. Chem. 2005, 72, 731–735. [Google Scholar] [CrossRef] [Green Version]
- Guven, O. An overview of current developments in applied radiation chemistry of polymers. In IAEA-Tec-Doc-1420 Advances in Radiation Chemistry of Polymers; International Atomic Energy Agency: Vienna, Austria, 2004; pp. 33–39. [Google Scholar]
- Casimiro, M.H.; Lancastre, J.J.; Rodrigues, A.P.; Gomes, S.R.; Rodrigues, G.; Ferreira, L.M. Chitosan-Based matrices prepared by gamma irradiation for tissue regeneration: Structural properties vs. preparation method. Top. Curr. Chem. 2017, 375. [Google Scholar] [CrossRef]
- McLaughlin, W.L.; Boyd, A.W.; Chadwick, K.H.; McDonald, J.C.; Miller, A. Dosimetry for Radiation Processing; Taylor and Francis: London, UK, 1989; pp. 1–251. ISBN 0-85066-740-2. [Google Scholar]
- Makhlis, F.A. Radiation Physics and Chemistry of Polymers, Halsted Press Book; John Wiley & Sons, Inc.: Jerusalem, Israel, 1975; pp. 1–287. ISBN 0-470-56537-3. [Google Scholar]
- Spinks, J.W.T.; Woods, R.J. An Introduction to Radiation Chemistry, 3rd ed.; John-Wiley and Sons, Inc.: Flatbush, NY, USA; Toronto, ON, Canada, 1990; ISBN 0-471-61403-3. [Google Scholar]
- Quinn, J.F.; Davis, T.P.; Barner, L.; Barner-Kowollik, C. The application of ionizing radiation in reversible addition-fragmentation chain transfer (RAFT) polymerization: Renaissance of a key synthetic and kinetic tool. Polymer 2007, 48, 6447–6480. [Google Scholar] [CrossRef]
- Casimiro, M.H.; Leal, J.P.; Gil, M.H. Characterisation of gamma irradiated chitosan/pHEMA membranes for biomedical purposes. Nucl. Instrum. Methods Phys. Res. Sect. B 2005, 236, 482–487. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.M.; Rocha, J.M.; Andrade, M.E.; Gil, M.H. Preparation and characterization of polyethylene based graft copolymers. Applications in the immobilization of enzymes. Radiat. Phys. Chem. 1998, 52, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.M.; Falcao, A.N.; Gil, M.H. Modification of LDPE molecular structure by gamma irradiation for bioapplications. Nucl. Instrum. Methods B 2005, 236, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.M.; Falcao, A.N.; Gil, M.H. Elemental and topographic characterization of LDPE based copolymeric films obtained by gamma irradiation. Nucl. Instrum. Methods B 2007, 265, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.M.; Leal, J.P.; Rodrigues, P.A.; Alves, L.C.; Falcao, A.N.; Gil, M.H. Characterization of PE-g-HEMA films prepared by gamma irradiation through nuclear microprobe techniques. Radiat. Phys. Chem. 2012, 81, 1319–1323. [Google Scholar] [CrossRef]
- Casimiro, M.H.; Silva, A.G.; Pinto, J.V.; Ramos, A.M.; Vital, J.; Ferreira, L.M. Catalytic poly(vinyl alcohol) functionalized membranes obtained by gamma irradiation. Radiat. Phys. Chem. 2012, 16, 1314–1318. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Leal, J.P.; Casimiro, M.H.; Cruz, C.; Lancastre, J.J.; Falcao, A.N. Evidence of structural order recovery in LDPE based copolymers prepared by gamma irradiation. Radiat. Phys. Chem. 2014, 94, 31–35. [Google Scholar] [CrossRef]
- Walo, M.; Przybytniak, G.; Barsby, M.; Guven, O. Functionalization of poly(ester-urethane) surface by radiation-induced grafting of N-isopropylacrylamide using conventional and reversible addition-fragmentation chain transfer-mediated methods. Polym. Int. 2016, 65, 192–199. [Google Scholar] [CrossRef]
- Poster, D.L.; Tsinas, Z.; Robertson, J.W.; Pazos, I.; Catterton, D.; Postek, M.T.; Kasianowicz, J.J.; Al-Sheikhly, M. Radiation-induced synthesis and modification of soft nanomaterials for medical applications. In Proceedings of the Nanoengineering: Fabrication, Properties, Optics and Devices XV, San Diego, CA, USA, 20–23 August 2018. [Google Scholar] [CrossRef]
- Stannet, V.T. Radiation grafting—State-of-the-art. Radiat. Phys. Chem. 1990, 35, 82–87. [Google Scholar] [CrossRef]
- Gil, M.H.M. Immobilisation of Proteins, Enzymes and Cells onto Graft Copolymeric Substrates. Ph.D. Thesis, University of Leeds, Leeds, UK, 1983. [Google Scholar]
- Ferreira, L.M. Preparação de suportes poliméricos com aplicação prática na indústria, utilizando a técnica de copolimerização de enxerto por radiação gama. Chemistry Degree Thesis, Lisbon University, Lisbon, Portugal, 1994. [Google Scholar]
- Stannet, V.T. Grafting. Radiat. Phys. Chem. 1981, 18, 215–222. [Google Scholar] [CrossRef]
- Pinnau, I.; Freeman, B.D. Formation and Modification of Polymeric Membranes: Overview. In Membrane Formation and Modification ACS Symposium Series; Pinnau, F., Ed.; American Chemical Society: Washington, DC, USA, 1999; pp. 2–20. ISBN 9780841236042. [Google Scholar]
- Geise, G.M.; Hickner, M.A.; Logan, B.E. Ionic resistance and permselectivity tradeoffs in anion exchange membranes. ACS Appl. Mater. Interfaces 2013, 5, 10294–10301. [Google Scholar] [CrossRef]
- Güler, E.; Elizen, R.; Vermaas, D.A.; Saakes, M.; Nijmeijer, K. Performance-determining membrane properties in reverse electrodialysis. J. Membr. Sci. 2013, 446, 266–276. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Gohil, G.S.; Shahi, V.K. Recent developments on ion-exchange membranes and electro-membrane processes. Adv. Colloid Interface Sci. 2006, 119, 97–130. [Google Scholar] [CrossRef]
- Grot, W. Fluorinated Ionomers; Elsevier: Amsterdam, The Netherlands, 2011; Chapter 9; pp. 211–233. [Google Scholar] [CrossRef]
- Dlugolecki, P.; Nymeijer, K.; Metz, S.; Wessling, M. Current status of ion exchange membranes for power generation from salinity gradients. J. Membr. Sci. 2008, 319, 214–222. [Google Scholar] [CrossRef]
- Mei, Y.; Tang, C.Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination 2018, 425, 156–174. [Google Scholar] [CrossRef]
- Wijers, M.C.; Jin, M.; Wessling, M.; Strathmann, H. Supported liquid membranes modification with sulphonated poly (ether ether ketone). Permeability, selectivity and stability. J. Membr. Sci. 1998, 147, 117–130. [Google Scholar] [CrossRef]
- Hong, J.G.; Zhang, B.; Glabman, S.; Uzal, N.; Dou, X.; Zhang, H.; Wei, X.; Chen, Y. Potential ion exchange membranes and system performance in reverse electrodialysis for power generation: A review. J. Membr. Sci. 2015, 486, 71–88. [Google Scholar] [CrossRef]
- Galama, A.H.; Vermaas, D.A.; Veerman, J.; Saakes, M.; Rijnaarts, H.H.M.; Post, J.W.; Nijmeijer, K. Membrane resistance: The effect of salinity gradients over a cation exchange membrane. J. Membr. Sci. 2014, 467, 279–291. [Google Scholar] [CrossRef]
- Disabb-Miller, M.L.; Johnson, Z.D.; Hickner, M.A. Ion motion in anion and proton conducting triblock copolymers. Macromolecules 2013, 46, 949–956. [Google Scholar] [CrossRef]
- Sata, T. Preparation of ion exchange membranes. In Ion Exchange Membranes: Preparation, Characterization, Modification and Application; Sata, T., Ed.; Royal Society of Chemistry: Cambridge, UK, 2007; pp. 35–88. ISBN 978-0-85404-590-7. [Google Scholar] [CrossRef]
- Dlugolecki, P.; Gambier, A.; Nijmeijer, K.; Wessling, M. Practical potential of reverse electrodialysis as process for sustainable energy generation. Environ. Sci. Technol. 2009, 43, 6888–6894. [Google Scholar] [CrossRef] [PubMed]
- Długołęcki, P.; Ogonowski, P.; Metz, S.J.; Saakes, M.; Nijmeijer, K.; Wessling, M. On the resistances of membrane, diffusion boundary layer and double layer in ion exchange membrane transport. J. Membr. Sci. 2010, 349, 369–379. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Kozmai, A.E. Electrical equivalent circuit of an ion-exchange membrane system. Electrochim. Acta 2011, 56, 1262–1269. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, J.; Wang, P.; Wang, Z.; Shi, F.; Liu, H. Investigations on the interfacial capacitance and the diffusion boundary layer thickness of ion exchange membrane using electrochemical impedance spectroscopy. J. Membr. Sci. 2016, 502, 37–47. [Google Scholar] [CrossRef]
- Strathmann, H. Ion-exchange membrane separation processes. In Membrane Science and Technologies Series, 1st ed.; Strathmann, H., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 9, pp. 1–348. ISBN 9780444502360. [Google Scholar]
- Nia, C.; Wang, H.; Zhao, Q.; Liu, B.; Sun, Z.; Zhang, M.; Hua, W.; Liang, L. Crosslinking effect in nanocrystalline cellulose reinforced sulfonated poly (aryl ether ketone) proton exchange membranes. Solid State Ion. 2018, 323, 5–15. [Google Scholar] [CrossRef]
- Cheng, J.; He, G.; Zhang, F. A mini-review on anion exchange membranes for fuel cell applications: Stability issue and addressing strategies. Int. J. Hydrog. Energy 2015, 40, 7348–7360. [Google Scholar] [CrossRef]
- Jose, A.J.; Kappen, J.; Alagar, M. 2-Polymeric membranes: Classification, preparation, structure physiochemical, and transport mechanisms. In Woodhead Publishing Series in Biomaterials, Fundamental Biomaterials: Polymers; Thomas, S., Balakrishnan, P., Sreekala, M.S., Eds.; Woodhead Publishing, Elsevier: Amsterdam, The Netherlands, 2018; pp. 21–35. ISBN 978-0-08-102195-8. [Google Scholar]
- Nasef, M.M. Radiation-grafted membranes for polymer electrolyte fuel cells: Current trends and future directions. Chem. Rev. 2014, 114, 12278–12329. [Google Scholar] [CrossRef]
- Domènech, B.; Bastos-Arrieta, J.; Alonso, A.; Macanás, J.; Muñoz, M.; Muraviev, D.N. Bifunctional polymer-metal nanocomposite ion exchange materials. In Ion Exchange Technologies; Kilislioglu, A., Ed.; IntechOpen Limited: London, UK, 2012; Chapter 3; pp. 35–72. ISBN 978-953-51-0836-8. [Google Scholar] [CrossRef] [Green Version]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Perfluorosulfonic Acid Membranes for Fuel Cell and Electrolyser Applications. Available online: https://www.sigmaaldrich.com/technical-documents/articles/materials-science/perfluorosulfonic-acid-membranes.html#summary (accessed on 4 November 2019).
- Treharne, A.J.; Thomson, H.A.; Grossel, M.C.; Lotery, A.J. Developing methacrylate-based copolymers as an artificial Bruch’s membrane substitute. J. Biomed. Mater. Res. A 2012, 100, 2358–2364. [Google Scholar] [CrossRef] [PubMed]
- Fraco, M.C.; Elder, M.J. lntraocular lens materials and styles: A review. Aust. N. Z. J. Ophthalmol. 1997, 25, 255–263. [Google Scholar]
- Pérez-Vives, C. Biomaterial influence on intraocular lens performance: An overview. J. Ophthalmol. 2018, 2687385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamatialis, D.F.; Papenburg, B.J.; Girones, M.; Saiful, S.; Bettahalli, S.N.; Schmitmeier, S.; Wessling, M. Medical applications of membranes: Drug delivery, artificial organs and tissue engineering. J. Membr. Sci. 2008, 308, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Rosiak, J.; Janik, I.; Kadlubowski, S.; Kozicki, M.; Kujawa, P.; Stasica, P.; Ulanski, P. Nano-, micro- and macroscopic hydrogels synthesized by radiation technique. Nucl. Instrum. Methods. Phys. Res. Sect. B 2003, 208, 325–330. [Google Scholar] [CrossRef]
- Ulanski, P.; Janik, I.; Kadlubowski, S.; Kozicki, M.; Kujawa, P.; Pietrzak, M.; Stasica, P.; Rosiak, J.M. Polymeric biomaterials synthesized by radiation techniques—Current studies at IARC, Poland. Polym. Advan. Technol. 2002, 13, 951–959. [Google Scholar] [CrossRef]
- Oliveira, M.J.; Amato, V.S.; Lugao, A.B.; Parra, D.F. Hybrid hydrogels produced by ionizing radiation technique. Radiat. Phys. Chem. 2012, 81, 1471–1474. [Google Scholar] [CrossRef]
- Lopez-Barriguete, J.E.; Isoshima, T.; Bucio, E. Development and characterization of thermal responsivehydrogel films for biomedical sensor application. Mater. Res. Expr. 2018, 5, 045703. [Google Scholar] [CrossRef]
- De Oliveira, M.J.; Parra, D.F.; Amato, V.S.; Lugao, A.B. Hydrogel membranes of PVA1/clay by gamma radiation. Radiat. Phys. Chem. 2013, 84, 111–114. [Google Scholar] [CrossRef]
- An, J.-C. Synthesis of poly(vinyl pyrrolidone) nanohydrogels by the template-assisted ionizing radiation. J. Ind. Eng. Chem. 2009, 15, 148–152. [Google Scholar] [CrossRef]
- Betz, N.; Begue, J.; Goncalves, M.; Gionnet, K.; Deleris, G.; Le Moel, A. Functionalisation of PAA radiation grafted PVDF. Nucl. Instrum. Methods. Phys. Res. Sect. B 2003, 208, 434–441. [Google Scholar] [CrossRef]
- Casimiro, M.H.; Gil, M.H.; Leal, J.P. Drug release assays from new chitosan/pHEMA membranes obtained by gamma irradiation. Nucl. Instrum. Methods. Phys. Res. Sect. B 2007, 265, 406–409. [Google Scholar] [CrossRef] [Green Version]
- Stoica-Guzun, A.; Stroescu, M.; Tache, F.; Zaharescu, T.; Grosu, E. Effect of electron beam irradiation on bacterial cellulose membranes used as transdermal drug delivery systems. Nucl. Instrum. Methods Phys. Res. Sect. B 2007, 265, 434–438. [Google Scholar] [CrossRef]
- Casimiro, M.H.; Gil, M.H.; Leal, J.P. Suitability of gamma irradiated chitosan based membranes as matrix in drug release system. Int. J. Pharm. 2010, 395, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Contreras-García, A.; Alvarez-Lorenzo, C.; Taboada, C.; Concheiro, A.; Bucio, E. Stimuli-responsive networks grafted onto polypropylene for the sustained delivery of NSAIDs. Acta Biomater. 2011, 7, 996–1008. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar] [CrossRef]
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef] [Green Version]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of scaffolds for bone-tissue regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.A.; Ma, P.X. Nano-fibrous scaffolds for tissue engineering. Colloid Surf. B 2004, 39, 125–131. [Google Scholar] [CrossRef]
- Bondioli, E.; Fini, M.; Veronesi, F.; Giavaresi, G.; Tschon, M.; Cenacchi, G.; Cerasoli, S.; Giardino, R.; Melandri, D. Development and evaluation of a decellularized membrane from human dermis. J. Tissue Eng. Regen. Med. 2014, 8, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.M.; Li, D.X.; Chen, X.N.; Lu, J.; Fan, H.S.; Zhang, X.D. Preparation and cytocompatibility of chitosan-modified polylactide. J. Appl. Polym. Sci. 2008, 110, 408–412. [Google Scholar] [CrossRef]
- Lee, S.H.; An, S.J.; Lim, Y.M.; Huh, J.B. The efficacy of electron beam irradiated bacterial cellulose membranes as compared with collagen membranes on guided bone regeneration in peri-implant bone defects. Materials 2017, 10, 1018. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, A.; Bekhit, A.E.A.; Sun, Z.F.; Ali, M.A. Bio-scaffolds produced from irradiated squid pen and crab chitosan with hydroxyapatite/beta-tricalcium phosphate for bone-tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, K.; Qureshi, S.W.; Afzal, S.; Gul, R.; Yar, M.; Kaleem, M.; Khan, A.S. Microwave-assisted synthesis and evaluation of type 1 collagen-apatite composites for dental tissue regeneration. J. Biomater. Appl. 2018, 33, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casimiro, M.H.; Gomes, S.R.; Rodrigues, G.; Leal, J.P.; Ferreira, L.M. Chitosan/Poly(vinylpyrrolidone) matrices obtained by gamma-irradiation for skin scaffolds: Characterization and preliminary cell response studies. Materials 2018, 11, 2535. [Google Scholar] [CrossRef] [Green Version]
- Mozalewska, W.; Czechowska-Biskup, R.; Olejnik, A.C.; Wach, R.A.; Ulanski, P.; Rosiak, J.M. Chitosan-containing hydrogel wound dressings prepared by radiation technique. Radiat. Phys. Chem. 2017, 134, 1–7. [Google Scholar] [CrossRef]
- NASA. Global Climate Change, Vital Signs of the Planet. Available online: https://climate.nasa.gov/vital-signs/carbon-dioxide/ (accessed on 28 May 2019).
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Budischak, C.; Sewell, D.; Thomson, H.; Mach, L.; Veron, D.E.; Kempton, W. Cost-minimized combinations of wind power, solar power and electrochemical storage, powering the grid up to 99.9% of the time. J. Power Sources 2013, 225, 60–74. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Santos, A.R.; Drache, M.; Ke, X.; Gohs, U.; Turek, T.; Becker, M.; Kunz, U.; Beuermann, S. Polymer electrolyte membranes prepared by pre-irradiation induced graft copolymerization on ETFE for vanadium redox flow battery applications. J. Membr. Sci. 2017, 524, 419–427. [Google Scholar] [CrossRef]
- Hoogers, G. Fuel Cell Technology—Hand Book; CRC Press: Boca Raton, FL, USA, 2003; pp. 1–27. ISBN 0-8493-0877-1. [Google Scholar]
- Ye, Y.-S.; Rick, J.; Hwang, B.-J. Water soluble polymers as proton exchange membranes for fuel cells. Polymers 2012, 4, 913–963. [Google Scholar] [CrossRef] [Green Version]
- Nasef, M.M.; Hegazy, E.-S.A. Preparation and applications of ion exchange membranes by radiation-induced graft copolymerization of polar monomers onto non-polar films. Prog. Polym. Sci. 2004, 29, 499–561. [Google Scholar] [CrossRef]
- Ma, J.; Peng, J.; Zhai, M. Radiation-grafted membranes for applications in renewable energy technology. In Radiation Technology for Advanced Materials from Basic to Modern Applications; Wu, G., Zhai, M., Wang, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 7; pp. 207–247. [Google Scholar] [CrossRef]
- Dekel, D.R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]
- Zhu, L.; Peng, X.; Shang, S.-L.; Kwasny, M.T.; Zimudzi, T.J.; Yu, X.; Saikia, N.; Pan, J.; Liu, Z.-K.; Tew, G.N.; et al. High performance anion exchange membrane fuel cells enabled by fluoropoly(olefin) membranes. Adv. Funct. Mater. 2019. [Google Scholar] [CrossRef]
- Nasef, M.M.; Gürsel, S.A.; Karabelli, D.; Güven, O. Radiation-grafted materials for energy conversion and energy storage applications. Prog. Polym. Sci. 2016, 63, 1–41. [Google Scholar] [CrossRef]
- Feng, S.; Pang, J.; Yu, X.; Wang, G.; Manthiram, A. High-performance semicrystalline poly (ether ketone)-based proton exchange membrane. ACS Appl. Mater. Interfaces 2017, 9, 24527–24537. [Google Scholar] [CrossRef]
- Colmati, F.; Alonso, C.G.; Martins, T.D.; de Lima, R.B.; Ribeiro, A.C.C.; Carvalho, L.L.; Sampaio, A.M.B.S.; Magalhães, M.M.; Coutinho, J.W.D.; de Souza, G.A.; et al. Production of hydrogen and their use in proton exchange membrane fuel cells. In Advances in Hydrogen Generation Technologies; Eyvaz, M., Ed.; IntechOpen Limited: London, UK, 2018; Chapter 4; pp. 63–78. ISBN 978-1-78923-535-7. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.K.W.; Mesrobian, R.B.; Metz, D.J.; Ballantine, D.S.; Glines, A. Graft copolymers derived by ionizing radiation. J. Polym. Sci. 1957, 23, 903–913. [Google Scholar] [CrossRef]
- Prakash, O.; Jana, K.K.; Manohar, M.; Shahi, V.K.; Khan, S.A.; Avasthic, D.; Maiti, P. Fabrication of a low-cost functionalized poly(vinylidene fluoride) nanohybrid membrane for superior fuel cells. Sustain. Energy Fuels 2019, 3, 1269–1282. [Google Scholar] [CrossRef]
- Sadeghia, S.; Şanlıb, L.I.; Gülerb, E.; Gürsel, S.A. Enhancing proton conductivity via sub-micron structures in proton conducting membranes originating from sulfonated PVDF powder by radiation-induced grafting. Solid State Ion. 2018, 314, 66–73. [Google Scholar] [CrossRef]
- Meyer, Q.; Zeng, Y.; Zhao, C. In situ and operando characterization of proton exchange membrane fuel cells. Adv. Mater. 2019, 31. [Google Scholar] [CrossRef]
- Gottesfeld, S.; Dekel, D.R.; Page, M.; Bae, C.; Yan, Y.S.; Zelenay, P.; Kim, Y.S. Anion exchange membrane fuel cells: Current status and remaining challenges. J. Power Sources 2018, 375, 170–184. [Google Scholar] [CrossRef]
- Matanovic, I.; Maurya, S.; Park, E.J.; Jeon, J.Y.; Bae, C.; Kim, Y.S. Adsorption of polyaromatic backbone impacts the performance of anion exchange membrane fuel cells. Chem. Mater. 2019, 31, 4195–4204. [Google Scholar] [CrossRef]
- Mamlouk, M.; Wang, X.; Scott, K.; Horsfall, J.A.; Williams, C. Characterization and application of anion exchange polymer membranes with non-platinum group metals for fuel cells. Proc. Inst. Mech. Eng. Part A 2011, 225, 152–160. [Google Scholar] [CrossRef]
- Wang, L.; Magliocca, E.; Cunningham, E.L.; Mustain, W.E.; Poynton, S.D.; Escudero-Cid, R.; Nasef, M.M.; Ponce-González, M.J.; Bance-Souahli, R.; Slade, R.C.T.; et al. An optimised synthesis of high performance radiation-grafted anion-exchange membranes. Green Chem. 2017, 19, 831–843. [Google Scholar] [CrossRef] [Green Version]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Sheng, W.C.; Gasteiger, H.A.; Shao-Horn, Y. Hydrogen oxidation and evolution reaction kinetics on platinum: Acid vs. alkaline electrolytes. J. Electrochem. Soc. 2010, 157, B1529–B1536. [Google Scholar] [CrossRef]
- Li, D.; Chung, H.T.; Maurya, S.; Matanovic, I.; Kim, Y.S. Impact of ionomer adsorption on alkaline hydrogen oxidation activity and fuel cell performance. Curr. Opin. Electrochem. 2018, 12, 189–195. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; St-Pierre, J. Study of the aromatic hydrocarbons poisoning of platinum cathodes on proton exchange membrane fuel cell spatial performance using a segmented cell system. J. Power Sources 2016, 333, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Kusoglu, A. Ionomer thin films in PEM fuel cells. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer Science+Business Media: Berlin, Germany, 2018; pp. 417–438. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, Y.S. Quaternized aryl ether-free polyaromatics for alkaline membrane fuel cells: Synthesis, properties, and performance—A topical review. J. Mater. Chem. A 2018, 6, 15456–15477. [Google Scholar] [CrossRef]
- Vahdat, A.; Bahrami, H.; Ansari, N.; Ziaie, F. Radiation grafting of styrene onto polypropylene fibres by a 10 MeV electron beam. Radiat. Phys. Chem. 2007, 76, 787–793. [Google Scholar] [CrossRef]
- Espiritu, R.; Mamlouk, M.; Scott, K. Study on the effect of the degree of grafting on the performance of polyethylene-based anion exchange membrane for fuel cell application. Int. J. Hydrog. Energy 2016, 41, 1120–1133. [Google Scholar] [CrossRef] [Green Version]
- Varcoe, J.R.; Slade, R.C.T.; Yee, E.L.H.; Poynton, S.D.; Driscoll, D.J.; Apperley, D.C. Poly(ethylene-co-tetrafluoroethylene)-derived radiation-grafted anion-exchange membrane with properties specifically tailored for application in metal-cation-free alkaline polymer electrolyte fuel cells. Chem. Mater. 2007, 19, 2686–2693. [Google Scholar] [CrossRef]
- Walsby, N.; Paronen, M.; Juhanoja, J.; Sundholm, F. Radiation grafting of styrene onto poly (vinylidene fluoride) films in propanol: The influence of solvent and synthesis conditions. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1512–1519. [Google Scholar] [CrossRef]
- Slade, R.C.T.; Varcoe, J.R. Investigations of conductivity in FEP-based radiation-grafted alkaline anion-exchange membranes. Solid State Ion. 2005, 176, 585–597. [Google Scholar] [CrossRef] [Green Version]
- Poynton, S.D.; Varcoe, J.R. Reduction of the monomer quantities required for the preparation of radiation-grafted alkaline anion-exchange membranes. Solid State Ion. 2015, 277, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Gubler, L.; Prost, N.; Gursel, S.A.; Scherer, G.G. Proton exchange membranes prepared by radiation grafting of styrene/divinylbenzene onto poly(ethylene-alt-tetrafluoroethylene) for low temperature fuel cells. Solid State Ion. 2005, 176, 2849–2860. [Google Scholar] [CrossRef]
- Schmidt-Naake, G.; Bohme, M.; Cabrera, A. Synthesis of proton exchange membranes with pendent phosphonic acid groups by irradiation grafting of VBC. Chem. Eng. Technol. 2005, 28, 720–724. [Google Scholar] [CrossRef]
- Nuñez, S.A.; Capparelli, C.; Hickner, M.A. N-Alkyl Interstitial spacers and terminal pendants influence the alkaline stability of tetraalkylammonium cations for anion exchange membrane fuel cells. Chem. Mater. 2016, 28, 2589–2598. [Google Scholar] [CrossRef]
- Sturgeon, M.R.; Macomber, C.S.; Engtrakul, C.; Long, H.; Pivovar, B.S. Hydroxide based benzyltrimethylammonium degradation: Quantification of rates and degradation technique development. J. Electrochem. Soc. 2015, F366–F372. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, A.D.; Bae, C. Mechanistic analysis of ammonium cation stability for alkaline exchange membrane fuel cells. J. Mater. Chem. A 2014, 2, 17314–17320. [Google Scholar] [CrossRef]
- Jannasch, P.; Weiber, E.A. Configuring anion-exchange membranes for high conductivity and alkaline stability by using cationic polymers with tailored side chains. Macromol. Chem. Phys. 2016, 217, 1108–1118. [Google Scholar] [CrossRef]
- Ponce-González, J.; Ouachan, I.; Varcoe, J.R.; Whelligan, D.K. Radiation-induced grafting of a butyl-spacer styrenic monomer onto ETFE: The synthesis of the most alkali stable radiation-grafted anion-exchange membrane to date. J. Mater. Chem. A 2018, 6, 823–827. [Google Scholar] [CrossRef] [Green Version]
- Ponce-Gonzalez, J.; Whelligan, D.K.; Wang, L.; Bance-Soualhi, R.; Wang, Y.; Peng, Y.; Peng, H.; Apperley, D.C.; Sarode, H.N.; Pandey, T.P.; et al. High performance aliphatic-heterocyclic benzyl-quaternary ammonium radiation-grafted anion-exchange membranes. Energy Environ. Sci. 2016, 9, 3724–3735. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Peng, X.; Mustain, W.E.; Varcoe, J.R. Radiation-grafted anion-exchange membranes: The switch from low- to high-density polyethylene leads to remarkably enhanced fuel cell performance. Energy Environ. Sci. 2019, 12, 1575–1579. [Google Scholar] [CrossRef] [Green Version]
- Omasta, T.J.; Park, A.M.; LaManna, J.M.; Zhang, Y.; Peng, X.; Wang, L.; Jacobson, D.L.; Varcoe, J.R.; Hussey, D.S.; Pivovar, B.S.; et al. Beyond catalysis and membranes: Visualizing and solving the challenge of electrode water accumulation and flooding in AEMFCs. Energy Environ. Sci. 2018, 11, 551–558. [Google Scholar] [CrossRef] [Green Version]
- Winsberg, J.; Hagemann, T.; Janoschka, T.; Hager, M.D.; Schubert, U.S. Redox-Flow Batteries: From Metals to Organic Redox-Active Materials. Angew. Chem. Int. Ed. 2017, 56, 686–711. [Google Scholar] [CrossRef] [PubMed]
- Alstone, P.; Gershenson, D.; Kammen, D.M. Decentralized energy systems for clean electricity access. Nat. Clim. Chang. 2015, 5, 305–314. [Google Scholar] [CrossRef]
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of current development in electrical energy storage technologies and the application potential in power system operation. Appl. Energy 2015, 137, 511–536. [Google Scholar] [CrossRef] [Green Version]
- Arenas, L.F.; Ponce de León, C.; Walsh, F.C. Engineering aspects of the design, construction and performance of modular redox flow batteries for energy storage. J. Energy Storage 2017, 11, 119–153. [Google Scholar] [CrossRef] [Green Version]
- Gubler, L. Membranes and separators for redox flow batteries. Curr. Opin. Electrochem. 2019, 18, 31–36. [Google Scholar] [CrossRef]
- Abdiania, M.; Abouzari-Lotf, E.; Ting, T.M.; Nia, P.M.; Sha’rani, S.S.; Shockravi, A.; Ahmad, A. Novel polyolefin based alkaline polymer electrolyte membrane for vanadium redox flow batteries. J. Power Sources 2019, 424, 245–253. [Google Scholar] [CrossRef]
- Jiang, B.; Wu, L.; Yu, L.; Qiu, X.; Xi, J. A comparative study of Nafion series membranes for vanadium redox flow batteries. J. Membr. Sci. 2016, 510, 18–26. [Google Scholar] [CrossRef]
- Sharma, P.P.; Paul, A.; Srivastava, D.N.; Kulshrestha, V. Semi-interpenetrating network-type cross-linked amphoteric ion-exchange membrane based on styrene sulfonate and vinyl benzyl chloride for vanadium redox flow battery. ACS Omega 2018, 3, 9872–9879. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Chen, X.; Wang, Y.; Peng, J.; Zhao, L.; Du, J.; Zhai, M. Amphoteric ion exchange membranes prepared by preirradiation-induced emulsion graft copolymerization for vanadium redox flow battery. Polymers 2019, 11, 1482. [Google Scholar] [CrossRef] [Green Version]
- Nasef, M.M.; Güven, O. Radiation-grafted copolymers for separation and purification purposes: Status, challenges and future direction. Prog. Polym. Sci. 2012, 37, 1597–1656. [Google Scholar] [CrossRef]
- Chowdhury, M.N.K.; Khan, M.W.; Mina, M.F.; Beg, M.D.H.; Khan, M.R.; Alam, A.K.M.M. Synthesis and characterization of radiation grafted films for removal of arsenic and some heavy metals from contaminated water. Radiat. Phys. Chem. 2012, 81, 1606–1611. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Rehim, H.A.; Hegazy, E.A.; El-Hag Ali, A. Selective removal of some heavy metal ions from aqueous solution using treated polyethylene-g-styrene/maleic anhydride membranes. React. Funct. Polym. 2000, 1, 105–116. [Google Scholar] [CrossRef]
- Ajji, Z.; Ali, A.M. Separation of copper ions from iron ions using PVA-g-(acrylic acid/N-vinyl imidazole) membranes prepared by radiation-induced grafting. J. Hazard. Mater. 2010, 1, 71–74. [Google Scholar] [CrossRef]
- Wang, Y.C.; Peng, J.; Li, J.Q.; Zhai, M.L. PVDF based ion exchange membrane prepared by radiation grafting of ethyl styrenesulfonate and sequent hydrolysis. Radiat. Phys. Chem. 2017, 130, 252–258. [Google Scholar] [CrossRef]
- Masuelli, M.A.; Grasselli, M.; Marchese, J.; Ochoa, N.A. Preparation, structural and functional characterization of modified porous PVDF membranes by gamma-irradiation. J. Membr. Sci. 2012, 389, 91–98. [Google Scholar] [CrossRef]
- Kwak, N.S.; Hwang, T.S.; Kim, S.M.; Yang, Y.K.; Kang, K.S. Synthesis of sulfonated PET-g-GMA fine ion-exchange fibers for water treatment by photopolymerization and their adsorption properties for metal ions. Polymer-Korea 2004, 28, 397–403. [Google Scholar]
- Ma, H.-L.; Zhang, Y.; Zhang, L.; Wang, L.; Sun, C.; Liu, P.; He, L.; Zeng, X.; Zhai, M. Radiation-induced graft copolymerization of dimethylaminoethyl methacrylate onto graphene oxide for Cr(VI) removal. Radiat. Phys. Chem. 2016, 124, 159–163. [Google Scholar] [CrossRef]
- Shin, I.H.; Hong, S.; Lim, S.J.; Son, Y.-S.; Kim, T.-H. Surface modification of PVDF membrane by radiation-induced graft polymerization for novel membrane bioreactor. J. Ind. Eng. Chem. 2017, 46, 103–110. [Google Scholar] [CrossRef]
- Bazante-Yamaguishi, R.; Moura, E.; Manzoli, J.E.; Geraldo, A.B.C. Radiation-grafted, chemically modified membranes part I—Synthesis of a selective aluminum material. Radiat. Phys. Chem. 2014, 94, 133–136. [Google Scholar] [CrossRef]
- Saito, K.; Sugo, T. High-performance polymeric materials for separation and reaction, prepared by radiation-induced graft polymerization. In Studies in Physical and Theoretical Chemistry; Charles, D.J., Rao, B.S.M., Eds.; Elsevier: Oxford, UK, 2001; pp. 671–704. [Google Scholar]
- Takeda, T.; Tamada, M.; Seko, N.; Ueki, Y. Ion exchange fabric synthesized by graft polymerization and its application to ultra-pure water production. Radiat. Phys. Chem. 2010, 130, 223–226. [Google Scholar] [CrossRef]
- Kaur, I.; Misra, B.N.; Kohli, A. Synthesis of Teflon-FEP grafted membranes for use in water desalination. Desalination 2001, 1, 357–365. [Google Scholar] [CrossRef]
- Tanaka, Y. Mass transport and energy consumption in ion-exchange membrane electrodialysis of seawater. J. Membr. Sci. 2003, 1, 265–279. [Google Scholar] [CrossRef]
- Bahal, M.; Kaur, N.; Sharotri, N.; Sud, D. Investigations on Amphoteric Chitosan/TiO2 Bionanocomposites for Application in Visible Light Induced Photocatalytic Degradation. Adv. Polym. Sci. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Mustafai, F.A.; Balouch, A.; Abdullah Jalbani, N.; Bhanger, M.I.; Jagirani, M.S.; Kumar, A.; Tunio, A. Microwave-assisted synthesis of imprinted polymer for selective removal of arsenic from drinking water by applying Taguchi statistical method. Eur. Polym. J. 2018, 109, 133–142. [Google Scholar] [CrossRef]












© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casimiro, M.H.; Ferreira, L.M.; Leal, J.P.; Pereira, C.C.L.; Monteiro, B. Ionizing Radiation for Preparation and Functionalization of Membranes and Their Biomedical and Environmental Applications. Membranes 2019, 9, 163. https://doi.org/10.3390/membranes9120163
Casimiro MH, Ferreira LM, Leal JP, Pereira CCL, Monteiro B. Ionizing Radiation for Preparation and Functionalization of Membranes and Their Biomedical and Environmental Applications. Membranes. 2019; 9(12):163. https://doi.org/10.3390/membranes9120163
Chicago/Turabian StyleCasimiro, Maria Helena, Luis Mota Ferreira, João Paulo Leal, Claudia Cristina Lage Pereira, and Bernardo Monteiro. 2019. "Ionizing Radiation for Preparation and Functionalization of Membranes and Their Biomedical and Environmental Applications" Membranes 9, no. 12: 163. https://doi.org/10.3390/membranes9120163
APA StyleCasimiro, M. H., Ferreira, L. M., Leal, J. P., Pereira, C. C. L., & Monteiro, B. (2019). Ionizing Radiation for Preparation and Functionalization of Membranes and Their Biomedical and Environmental Applications. Membranes, 9(12), 163. https://doi.org/10.3390/membranes9120163