Comparison of Current International Guidelines for the Management of Dyslipidemia
Abstract
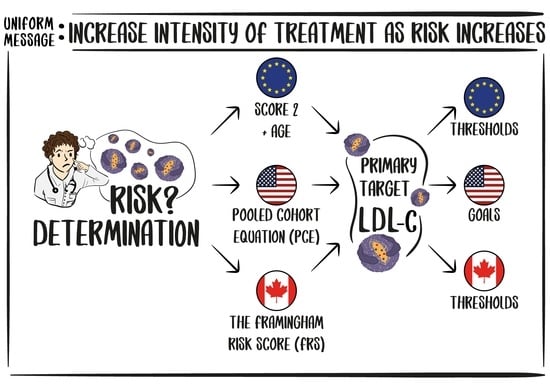
:1. Introduction
2. Risk Estimation Tools and Definition of Risk Categories
3. Risk Modifiers and Risk-Enhancing Factors
4. Lipid Measurement
5. Primary Prevention
6. Secondary Prevention
7. Very High-Risk Patients
8. Familial Hypercholesterolemia
9. Other Specific Groups
9.1. Diabetes Mellitus
9.2. Chronic Kidney Disease
9.3. Hypertriglyceridemia
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parini, P.; Frikke-Schmidt, R.; Tselepis, A.D.; Moulin, P.; von Eckardstein, A.; Binder, C.J.; Catapano, A.L.; Ray, K.K.; Tokgözoğlu, L. Taking action: European Atherosclerosis Society targets the United Nations Sustainable Development Goals 2030 agenda to fight atherosclerotic cardiovascular disease in Europe. Atherosclerosis 2021, 322, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Tokgözoğlu, L. Chasing LDL cholesterol to the bottom—PCSK9 in perspective. Nat. Cardiovasc. Res. 2022, 1, 554–561. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart association task force on clinical practice guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [PubMed]
- Visseren, F.L.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar]
- Pearson, G.J.; Thanassoulis, G.; Anderson, T.J.; Barry, A.R.; Couture, P.; Dayan, N.; Francis, G.A.; Genest, J.; Grégoire, J.; Grover, S.A.; et al. Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in Adults. Can. J. Cardiol. 2021, 37, 1129–1150. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Coupland, C.; Brindle, P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ 2017, 357, j2099. [Google Scholar] [CrossRef] [Green Version]
- Miida, T.; Nishimura, K.; Okamura, T.; Hirayama, S.; Ohmura, H.; Yoshida, H.; Miyashita, Y.; Ai, M.; Tanaka, A.; Sumino, H.; et al. A multicenter study on the precision and accuracy of homogeneous assays for LDL-cholesterol: Comparison with a beta-quantification method using fresh serum obtained from non-diseased and diseased subjects. Atherosclerosis 2012, 225, 208–215. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Williams, K.; Contois, J.H.; Monroe, H.M.; McQueen, M.J.; de Graaf, J.; Furberg, C.D. A meta-analysis of lowdensity lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Kamstrup, P.R.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Extreme lipoprotein(a) levels and improved cardiovascular risk prediction. J. Am. Coll. Cardiol. 2013, 61, 1146–1156. [Google Scholar] [CrossRef] [Green Version]
- Van der Valk, F.M.; Bekkering, S.; Kroon, J.; Yeang, C.; Van den Bossche, J.; van Buul, J.D.; Ravandi, A.; Nederveen, A.J.; Verberne, H.J.; Scipione, C.; et al. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation 2016, 134, 611–624. [Google Scholar] [CrossRef] [Green Version]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar]
- Writing Committee; Lloyd-Jones, D.M.; Morris, P.B.; Ballantyne, C.M.; Birtcher, K.K.; Covington, A.M.; DePalma, S.M.; Minissian, M.B.; Orringer, C.E.; Smith, S.C.; et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: A report of the American College of Cardiology solution set oversight committee. J. Am. Coll. Cardiol. 2022, 80, 1366–1418. [Google Scholar] [PubMed]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- McGowan, M.P.; Hosseini Dehkordi, S.H.; Moriarty, P.M.; Duell, P.B. Diagnosis and treatment of heterozygous familial hypercholesterolemia. J. Am. Heart Assoc. 2019, 8, e013225. [Google Scholar] [CrossRef]
- Virani, S.S.; Morris, P.B.; Agarwala, A.; Ballantyne, C.M.; Birtcher, K.K.; Kris-Etherton, P.M.; Ladden-Stirling, A.B.; Miller, M.; Orringer, C.E.; Stone, N.J. 2021 ACC expert consensus decision pathway on the management of ASCVD risk reduction in patients with persistent hypertriglyceridemia: A report of the American college of cardiology solution set oversight committee. J. Am. Coll. Cardiol. 2021, 78, 960–993. [Google Scholar] [CrossRef] [PubMed]
- Orringer, C.E.; Tokgozoglu, L.; Maki, K.C.; Ray, K.K.; Saseen, J.J.; Catapano, A.L. Transatlantic lipid guideline divergence: Same data but different interpretations. J. Am. Heart Assoc. 2020, 9, e018189. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Giugliano, R.P.; Wiviott, S.D.; Atar, D.; Keech, A.; Kuder, J.F.; Im, K.; Murphy, S.A.; Flores-Arredondo, J.H.; López, J.A.; et al. Long-term evolocumab in patients with established atherosclerotic cardiovascular disease. Circulation 2022, 146, 1109–1119. [Google Scholar] [CrossRef]
- Kotseva, K.; Wood, D.; Backer, G.D.; Bacquer, D.D.; Pyörälä, K.; Keil, U.; EUROASPIRE Study Group. EUROASPIRE III: A survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countries. Eur. J. Cardiovasc. Prev. Rehabil. 2009, 16, 121–137. [Google Scholar] [CrossRef]
- Kotseva, K.; Wood, D.; De Bacquer, D.; De Backer, G.; Rydén, L.; Jennings, C.; Gyberg, V.; Amouyel, P.; Bruthans, J.; Castro Conde, A.; et al. EUROASPIRE IV: A European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur. J. Prev. Cardiol. 2016, 23, 636–648. [Google Scholar] [CrossRef]
- De Backer, G.; Jankowski, P.; Kotseva, K.; Mirrakhimov, E.; Reiner, Ž.; Ryden, L.; Tokgözoğlu, L.; Wood, D.; De Bacquer, D.; De Backer, G.; et al. Management of dyslipidaemia in patients with coronary heart disease: Results from the ESC-EORP EUROASPIRE V survey in 27 countries. Atherosclerosis 2019, 285, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Ray, K.K.; Molemans, B.; Schoonen, W.M.; Giovas, P.; Bray, S.; Kiru, G.; Murphy, J.; Banach, M.; De Servi, S.; Gaita, D.; et al. EU-wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: The DA VINCI study. Eur. J. Prev. Cardiol. 2021, 28, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Tokgozoglu, L.; Torp-Pedersen, C. Redefining cardiovascular risk prediction: Is the crystal ball clearer now? Eur. Heart J. 2021, 42, 2468–2471. [Google Scholar] [CrossRef]
- Dainis, A.; Ashley, E. Cardiovascular precision medicine in the genomics era. J. Am. Coll. Cardiol. Basic Trans. Sci. 2018, 3, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Landstrom, A.P.; Kim, J.J.; Gelb, B.D.; Helm, B.M.; Kannankeril, P.J.; Semsarian, C.; Sturm, A.C.; Tristani-Firouzi, M.; Ware, S.M.; American Heart Association Council on Genomic and Precision Medicine; et al. Genetic testing for heritable cardiovascular diseases in pediatric patients: A scientific statement from the American Heart Association. Circ. Genom. Precis. Med. 2021, 14, e000086. [Google Scholar] [CrossRef] [PubMed]
| Risk Categories | Countries |
|---|---|
| Low-risk | Belgium, Denmark, France, Israel, Luxembourg, Norway, Spain, the Netherlands, the United Kingdom, Switzerland |
| Moderate-risk | Austria, Cyprus, Finland, Germany, Greece, Iceland, Ireland, Italy, Malta, Portugal, San Marino, Slovenia, and Sweden |
| High-risk | Albania, Bosnia and Herzegovina, Croatia, Czech Republic, Estonia, Hungary, Kazakhstan, Poland, Slovakia, and Turkey |
| Very high-risk | Algeria, Armenia, Azerbaijan, Belarus, Bulgaria, Egypt, Georgia, Kyrgyzstan, Latvia, Lebanon, Libya, Lithuania, Montenegro, Morocco, Republic of Moldova, Romania, Russian Federation, Serbia, Syria, The Former Yugoslav Republic (Macedonia), Tunisia, Ukraine, and Uzbekistan |
| ESC GUIDELINES | AHA/ACC/MS GUIDELINES | CCS GUIDELINES | |
|---|---|---|---|
| RISK CATEGORIES | 10-year SCORE2/SCORE2-OP percentages (fatal and non-fatal CVD risk) <50 years: <2.5%, 2.5–7.5%, ≥7.5% 50–69 years: <5%, 5–10%, ≥10% ≥70 years: <7.5%, 7.5–15%, ≥15% (Low-to-moderate-risk, high-risk and very high-risk, respectively) | 10-year risk ASCVD percentages (fatal and non-fatal ASCVD) High: ≥20% Intermediate: ≥7.5–<20% Borderline: 5–<7.5% Low: <5% | FRS 10-year CHD RİSK Low-risk FRS: <10% Intermediate-risk FRS: 10–19.9% or LDL-C ≥ 3.5 mmol/L or Non-HDL-C ≥ 4.2 mmol/L or ApoB ≥ 1.05 g/L or Men ≥ 50 and women ≥ 60 years with additional risk factors or with presence of other risk modifiers High-risk FRS: ≥ 20% |
| ESC Risk Modifiers | AHA/ACC/MS Risk-Enhancing Factors | CCS Risk Modifiers |
|---|---|---|
| Family history of premature CVD (men: <55 years and women: <60 years) | Family history of premature ASCVD (males: <55 years; females: <65 years) | Family history of premature coronary artery disease |
| Obesity and central obesity | ABI < 0.9 | Abdominal obesity |
| Physical inactivity Social deprivation and psychosocial stress, including vital exhaustion. | High-risk race/ethnicities (e.g., South Asian ancestry) | Physical inactivity Psychosocial factors |
|
| Excessive alcohol consumption |
| Coronary Artery Calcium score [CAC] > 0 Agatston Units (AUs) | ||
| Sex-specific conditions: Pregnancy-related hypertension Preeclampsia/Eclampsia Erectile dysfunction | Biomarkers
| Biomarkers
|
| Sex-specific Conditions: Premature menopause (before age of 40) Pregnancy-associated conditions (preeclampsia, eclampsia) | Sex-pecific conditions: Pregnancy-related hypertension Preeclampsia/eclampsia |
| PRIMARY PREVENTION | SECONDARY PREVENTION | |
|---|---|---|
| ESC Guidelines | Despite maximally tolerated statin dosage, ≥50% LDL-C reduction from baseline and LDL-C goal of <1.4 mmol/L (55 mg/dL) in very high-risk groups, <1.8 mmol/L (<70 mg/dL) in high-risk groups, <2.6 mmol/L (<100 mg/dL) in moderate-risk groups <3.0 mmol/L (<116 mg/dL) in low-risk groups is not achieved, treatment intensification with non-statin agents is recommended. | If LDL-C ≥ 55 mg/dL, despite maximally tolerated statin dosage, addition of ezetimibe or PCSK9 inhibitors after ezetimibe initiation is recommended. |
| AHA/ACC/MS Guideline * | In adults without ASCVD or diabetes with LDL-C level of 70–189 mg/dL, if patient has ≥20% risk, and In adults with diabetes without ASCVD and with LDL-C < 190 mg/dL, if ≥50% reduction in LDL-C level or LDL-C < 70 mg/dL or non-HDL-C < 100 mg/dL are not achieved, despite statin therapy, ezetimibe additon may be reasonable. In adults without ASCVD and LDL-C ≥ 190 mg/dL, if ≥50% reduction in LDL-C level or LDL-C < 100 mg/dL or non-HDL-C < 130 mg/dL are not achieved, despite statin therapy, non-statin agents are recommended. | Patients with ASCVD and at very high-risk adults with ASCVD at very high-risk, if ≥50% reduction of LDL-C level or LDL-C < 55 mg/dL are not achieved despite statin therapy, non-statin agents are recommended. For patients with ASCVD but without very high- risk, if ≥50% reduction of LDL-C level or LDL-C < 70 mg/dL are not achieved despite statin therapy non-statin agents are recommended. |
| CCS Guideline | Despite maximally tolerated statin dose, LDL-C ≥ 2.0 mmol/L or ApoB ≥ 0.8 g/L or Non-HDL-C ≥ 2.6 mmol/L, ezetimibe and/or PCSK-9 inhibitors are recommended; Despite maximally tolerated statin dose with or without ezetimibe, for patients with heterozygous FH without clinical ASCVD, if LDL-C ≥ 2.5 mmol/L or <50% reduction from baseline; or ApoB ≥ 0.85 g/L or non-HDL-C ≥ 3.2 mmol/L) PCSK-9 inhibitors are recommended. | Despite maximally tolerated statin dose, LDL-C ≥ 1.8–2.2 mmol/L or ApoB ≥ 0.7–0.8 g/dL or Non-HDL-C ≥ 2.4–2.9 mmol/L PCSK9 inhibitors with or without ezetimibe ar recommended. Despite maximally tolerated statin dose, LDL-C ≥ 2.2 mmol/L or ApoB ≥ 0.8 g/L or Non-HDL-C ≥ 2.9 mmol/L, PCSK9 inhibitors with or without ezetimibe are recommended. |
| ESC GUIDELINES | AHA/ACC/MS GUIDELINES | CCS GUIDELINES |
|---|---|---|
| To have one of these conditions below | Two or more major ASCVD events OR One major event and >1 high-risk condition | To have one of these conditions below |
| • Documented clinical ASCVD • Unequivocal ASCVD on imaging predictive of ASCVD events • Type 2 diabetes mellitus with target organ damage (microalbuminuria, retinopathy, or neuropathy), or at least three major risk factors, or early onset T1DM of long duration (>20 y) • Severe CKD (eGFR < 30 mL/min per 1.73 m2). • A calculated SCORE ≥ 10% or 10-year risk of fatal CVD • FH with ASCVD or with another major risk factor | Major ASCVD events | Recent acute coronary event (ACS): • Hospitalized index ACS to 52 weeks post index ACS Clinically evident ASCVD and any of the following: • Diabetes mellitus or metabolic syndrome • Polyvascular disease (vascular disease in ≥2 arterial beds) • Symptomatic PAD • Recurrent MI • MI in the past 2 years • Previous CABG surgery • LDL-C ≥ 2.6 mmol/L or heterozygous FH • Lipoprotein(a) ≥ 60 mg/dL (120 nmol/L) High-risk conditions for primary prevention: • CKD • Diabetes mellitus in patients > 40 years or patients > 30 years and with 15 or more years’ duration of diabetes or with microvascular complications • Abdominal aortic aneurysm > 3.0 cm or previous aortic aneurysm surgery. |
| • Recent ACS (within the past 12 months) • History of MI (other than the recent ACS event listed above) • History of ischemic stroke • Symptomatic peripheral arterial disease (history of claudication with ABI <0.85, or previous revascularization or amputation) | ||
| High-risk conditions | ||
| • Age ≥ 65 years • Diabetes mellitus • Hypertension • CKD (eGFR 15–59 mL/min per 1.73 m2) • History of congestive heart failure • Current smoking • Heterozygous FH • History of prior coronary artery bypass surgery or percutaneous coronary intervention outside of the major ASCVD event(s) • Persistently elevated LDL-C ≥ 100 mg/dL (2.6 mmol/L), despite maximally tolerated statin therapy and ezetimibe |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aygun, S.; Tokgozoglu, L. Comparison of Current International Guidelines for the Management of Dyslipidemia. J. Clin. Med. 2022, 11, 7249. https://doi.org/10.3390/jcm11237249
Aygun S, Tokgozoglu L. Comparison of Current International Guidelines for the Management of Dyslipidemia. Journal of Clinical Medicine. 2022; 11(23):7249. https://doi.org/10.3390/jcm11237249
Chicago/Turabian StyleAygun, Sevda, and Lale Tokgozoglu. 2022. "Comparison of Current International Guidelines for the Management of Dyslipidemia" Journal of Clinical Medicine 11, no. 23: 7249. https://doi.org/10.3390/jcm11237249
APA StyleAygun, S., & Tokgozoglu, L. (2022). Comparison of Current International Guidelines for the Management of Dyslipidemia. Journal of Clinical Medicine, 11(23), 7249. https://doi.org/10.3390/jcm11237249