Factors Associated with Response to Systemic Corticosteroids in Active Ulcerative Colitis: Results from a Prospective, Multicenter Trial
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Data Collection
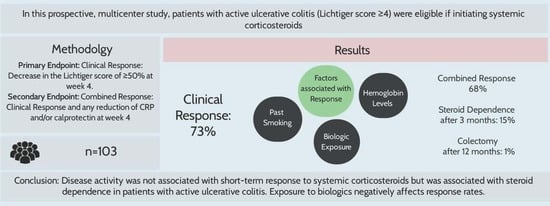
2.3. Primary Endpoint
2.4. Secondary Endpoints
2.5. Serum Samples, Fecal Calprotectin/Lipocalin-2
2.6. Statistical Analysis
3. Results
3.1. Clinical Efficacy of Systemic Corticosteroids in Active UC
3.2. Biochemical Efficacy of Systemic Corticosteroids in Active UC
3.3. Steroid Dependence and Colectomy Rates
3.4. Influence of Disease Activity on Response to Corticosteroids
3.5. Influence of Medical Therapies on Clinical Response to Corticosteroids
3.6. Factors Influencing Response to Corticosteroids
3.7. Modelling Response Prediction by Baseline Covariates
3.8. Sensitivity Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro-Sanchez, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohn’s Colitis 2021, 16, 2–17. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Isaacs, K.L.; Schneider, Y.; Siddique, S.M.; Falck-Ytter, Y.; Singh, S. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020, 158, 1450–1461. [Google Scholar] [CrossRef] [Green Version]
- Jeuring, S.F.G.; Biemans, V.B.C.; van den Heuvel, T.R.A.; Zeegers, M.P.; Hameeteman, W.H.; Romberg-Camps, M.J.L.; Oostenbrug, L.E.; Masclee, A.A.M.; Jonkers, D.; Pierik, M.J. Corticosteroid Sparing in Inflammatory Bowel Disease is More Often Achieved in the Immunomodulator and Biological Era-Results from the Dutch Population-Based IBDSL Cohort. Am. J. Gastroenterol. 2018, 113, 384–395. [Google Scholar] [CrossRef]
- Targownik, L.E.; Nugent, Z.; Singh, H.; Bernstein, C.N. Prevalence of and outcomes associated with corticosteroid prescription in inflammatory bowel disease. Inflamm. Bowel Dis. 2014, 20, 622–630. [Google Scholar] [CrossRef]
- Turner, D.; Walsh, C.M.; Steinhart, A.H.; Griffiths, A.M. Response to corticosteroids in severe ulcerative colitis: A systematic review of the literature and a meta-regression. Clin. Gastroenterol. Hepatol. 2007, 5, 103–110. [Google Scholar] [CrossRef]
- Truelove, S.C.; Watkinson, G.; Draper, G. Comparison of corticosteroid and sulphasalazine therapy in ulcerative colitis. Br. Med. J. 1962, 2, 1708–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lennard-Jones, J.E.; Longmore, A.J.; Newell, A.C.; Wilson, C.W.; Jones, F.A. An assessment of prednisone, salazopyrin, and topical hydrocortisone hemisuccinate used as out-patient treatment for ulcerative colitis. Gut 1960, 1, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Ben-Horin, S.; Har-Noy, O.; Katsanos, K.H.; Roblin, X.; Chen, M.; Gao, X.; Schwartz, D.; Cheon, J.H.; Cesarini, M.; Bojic, D.; et al. Corticosteroids and Mesalamine Versus Corticosteroids for Acute Severe Ulcerative Colitis: A Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2022, 20, 2868–2875.e1. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Leach, S.T.; Mack, D.; Uusoue, K.; McLernon, R.; Hyams, J.; Leleiko, N.; Walters, T.D.; Crandall, W.; Markowitz, J.; et al. Faecal calprotectin, lactoferrin, M2-pyruvate kinase and S100A12 in severe ulcerative colitis: A prospective multicentre comparison of predicting outcomes and monitoring response. Gut 2010, 59, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Sasson, A.N.; Shannon, K.M.; Ananthakrishnan, A.N. Fecal Calprotectin Is a Predictor of Need for Rescue Therapy in Hospitalized Severe Colitis. Inflamm. Bowel Dis. 2022, 28, 1833–1837. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, S.; Cassinotti, A.; Duca, P.; Mazzali, C.; Penati, C.; Manes, G.; Marmo, R.; Massari, A.; Molteni, P.; Maconi, G.; et al. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin. Gastroenterol. Hepatol. 2011, 9, 483–489.e483. [Google Scholar] [CrossRef] [PubMed]
- Blesl, A.; Wurm, P.; Waschina, S.; Gröchenig, H.P.; Novacek, G.; Primas, C.; Reinisch, W.; Kutschera, M.; Illiasch, C.; Hennlich, B.; et al. Prediction of Response to Systemic Corticosteroids in Active UC by Microbial Composition—A Prospective Multicenter Study. Inflamm. Bowel Dis. 2023. [Google Scholar] [CrossRef]
- Laharie, D.; Bourreille, A.; Branche, J.; Allez, M.; Bouhnik, Y.; Filippi, J.; Zerbib, F.; Savoye, G.; Nachury, M.; Moreau, J.; et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: A parallel, open-label randomised controlled trial. Lancet 2012, 380, 1909–1915. [Google Scholar] [CrossRef] [Green Version]
- D’haens, G.; Sandborn, W.J.; Feagan, B.G.; Geboes, K.; Hanauer, S.B.; Irvine, E.J.; Lémann, M.; Marteau, P.; Rutgeerts, P.; Schölmerich, J. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology 2007, 132, 763–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtiger, S.; Present, D.H. Preliminary report: Cyclosporin in treatment of severe active ulcerative colitis. Lancet 1990, 336, 16–19. [Google Scholar] [CrossRef]
- Lewis, J.D.; Chuai, S.; Nessel, L.; Lichtenstein, G.R.; Aberra, F.N.; Ellenberg, J.H. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm. Bowel Dis. 2008, 14, 1660–1666. [Google Scholar] [CrossRef] [Green Version]
- Sandborn, W.J.; Feagan, B.G.; Hanauer, S.; Vermeire, S.; Ghosh, S.; Liu, W.J.; Petersen, A.; Charles, L.; Huang, V.; Usiskin, K.; et al. Long-Term Efficacy and Safety of Ozanimod in Moderately to Severely Active Ulcerative Colitis: Results from the Open-Label Extension of the Randomized, Phase 2 TOUCHSTONE Study. J. Crohn’s Colitis 2021, 15, 1120–1129. [Google Scholar] [CrossRef]
- Hoffmann, P.; Globig, A.M.; Thomann, A.K.; Grigorian, M.; Krisam, J.; Hasselblatt, P.; Reindl, W.; Gauss, A. Tofacitinib in Treatment-Refractory Moderate to Severe Ulcerative Colitis: Real-World Experience from a Retrospective Multicenter Observational Study. J. Clin. Med. 2020, 9, 2177. [Google Scholar] [CrossRef]
- Samaan, M.A.; Cunningham, G.; Tamilarasan, A.G.; Beltran, L.; Pavlidis, P.; Ray, S.; Mawdsley, J.; Anderson, S.H.; Sanderson, J.D.; Arkir, Z.; et al. Therapeutic thresholds for golimumab serum concentrations during induction and maintenance therapy in ulcerative colitis: Results from the GO-LEVEL study. Aliment. Pharmacol. Ther. 2020, 52, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Colman, R.J.; Dhaliwal, J.; Rosen, M.J. Predicting Therapeutic Response in Pediatric Ulcerative Colitis—A Journey Towards Precision Medicine. Front. Pediatr. 2021, 9, 634739. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Visuri, I.; Vigren, L.; Nilsson, L.; Kärnell, A.; Hjortswang, H.; Bergemalm, D.; Almer, S.; Hertervig, E.; Karlén, P.; et al. Clinical effectiveness of golimumab in ulcerative colitis: A prospective multicentre study based on the Swedish IBD Quality Register, SWIBREG. Scand. J. Gastroenterol. 2021, 56, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Biemans, V.B.C.; Sleutjes, J.A.M.; de Vries, A.C.; Bodelier, A.G.L.; Dijkstra, G.; Oldenburg, B.; Löwenberg, M.; van Bodegraven, A.A.; van der Meulen-de Jong, A.E.; de Boer, N.K.H.; et al. Tofacitinib for ulcerative colitis: Results of the prospective Dutch Initiative on Crohn and Colitis (ICC) registry. Aliment. Pharmacol. Ther. 2020, 51, 880–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stange, E.F.; Travis, S.P.L.; Vermeire, S.; Reinisch, W.; Geboes, K.; Barakauskiene, A.; Feakins, R.; Fléjou, J.F.; Herfarth, H.; Hommes, D.W. European evidence-based consensus on the diagnosis and management of ulcerative colitis: Definitions and diagnosis. J. Crohn’s Colitis 2008, 2, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 19 July 2023).
- De Cristofaro, E.; Salvatori, S.; Marafini, I.; Zorzi, F.; Alfieri, N.; Musumeci, M.; Calabrese, E.; Monteleone, G. Long-Term Risk of Colectomy in Patients with Severe Ulcerative Colitis Responding to Intravenous Corticosteroids or Infliximab. J. Clin. Med. 2022, 11, 1679. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; D'Haens, G.; Wolf, D.C.; Jovanovic, I.; Hanauer, S.B.; Ghosh, S.; Petersen, A.; Hua, S.Y.; Lee, J.H.; et al. Ozanimod as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2021, 385, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Danese, S.; Loftus, E.V., Jr.; Vermeire, S.; Schreiber, S.; Ritter, T.; Fogel, R.; Mehta, R.; Nijhawan, S.; Kempiński, R.; et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): A phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet 2021, 397, 2372–2384. [Google Scholar] [CrossRef]
- Feagan, B.G.; Rubin, D.T.; Danese, S.; Vermeire, S.; Abhyankar, B.; Sankoh, S.; James, A.; Smyth, M. Efficacy of vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin. Gastroenterol. Hepatol. 2017, 15, 229–239.e225. [Google Scholar] [CrossRef] [Green Version]
- Amiot, A.; Filippi, J.; Abitbol, V.; Cadiot, G.; Laharie, D.; Serrero, M.; Altwegg, R.; Bouhnik, Y.; Peyrin-Biroulet, L.; Gilletta, C. Effectiveness and safety of ustekinumab induction therapy for 103 patients with ulcerative colitis: A GETAID multicentre real-world cohort study. Aliment. Pharmacol. Ther. 2020, 51, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- Bertani, L.; Blandizzi, C.; Mumolo, M.G.; Ceccarelli, L.; Albano, E.; Tapete, G.; Baiano Svizzero, G.; Zanzi, F.; Coppini, F.; de Bortoli, N.; et al. Fecal Calprotectin Predicts Mucosal Healing in Patients With Ulcerative Colitis Treated With Biological Therapies: A Prospective Study. Clin. Transl. Gastroenterol. 2020, 11, e00174. [Google Scholar] [CrossRef]
- Guidi, L.; Marzo, M.; Andrisani, G.; Felice, C.; Pugliese, D.; Mocci, G.; Nardone, O.; De Vitis, I.; Papa, A.; Rapaccini, G.; et al. Faecal calprotectin assay after induction with anti-Tumour Necrosis Factor α agents in inflammatory bowel disease: Prediction of clinical response and mucosal healing at one year. Dig. Liver Dis. 2014, 46, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Frin, A.C.; Filippi, J.; Boschetti, G.; Flourie, B.; Drai, J.; Ferrari, P.; Hebuterne, X.; Nancey, S. Accuracies of fecal calprotectin, lactoferrin, M2-pyruvate kinase, neopterin and zonulin to predict the response to infliximab in ulcerative colitis. Dig. Liver Dis. 2017, 49, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Kedia, S.; Bopanna, S.; Sachdev, V.; Sahni, P.; Dash, N.R.; Pal, S.; Vishnubhatla, S.; Makharia, G.; Travis, S.P.L.; et al. Faecal Calprotectin and UCEIS Predict Short-term Outcomes in Acute Severe Colitis: Prospective Cohort Study. J. Crohn’s Colitis 2017, 11, 1309–1316. [Google Scholar] [CrossRef] [PubMed]




| Endpoint | Definition |
|---|---|
| Primary endpoint | |
| Lichtiger response | Decrease in the Lichtiger score of ≥50% from baseline to week 4 |
| Secondary endpoints | |
| Lichtiger remission | Lichtiger score ≤ 3 after 4 weeks |
| Combined response | Decrease in the Lichtiger score of ≥50% from baseline to week 4 and any reduction of CRP and/or calprotectin in patients with elevated CRP or calprotectin at baseline |
| Combined remission | Lichtiger score ≤ 3 and CRP < 5 mg/L and/or calprotectin < 250 mg/kg after 4 weeks in patients with elevated CRP and/or calprotectin at baseline |
| Response (n = 75) | Nonresponse (n = 28) | p-Value | |
|---|---|---|---|
| Age (years) | 38 (20, 75) | 40 (21, 82) | 0.412 |
| Female sex | 33 (45) | 12 (43) | 0.875 |
| Body mass index | 23.6 (16.6, 34.8) | 24.5 (19.7, 36.8) | 0.126 |
| Disease duration at study inclusion (years) | 3 (0, 59) | 6 (0, 34) | 0.466 |
| Disease extent/Montreal classification | 0.344 | ||
| E1 | 8 (11) | 3 (11) | |
| E2 | 34 (45) | 8 (30) | |
| E3 | 33 (44) | 16 (59) | |
| Unknown | 1 | ||
| Severely active colitis | 36 (48) | 12 (43) | 0.642 |
| Hospitalization | 29 (41) | 12 (43) | 0.855 |
| Active smoking | 5 (7) | 4 (14) | 0.266 |
| Past smoking | 33 (46) | 21 (78) | 0.005 |
| Prednisolone dose (mg) | 50 (25, 75) | 50 (25, 100) | 0.416 |
| Methylprednisolone dose (mg) | 40 (32, 64) | 64 (48, 80) | 0.013 |
| Concomitant biologics | 11 (15) | 7 (25) | 0.249 |
| Concomitant immunomodulators | 4 (5) | 3 (11) | 0.386 |
| Concomitant oral 5-ASA | 56 (75) | 20 (71) | 0.740 |
| Prior use of corticosteroids | 32 (43) | 13 (46) | 0.732 |
| Hemoglobin (g/dL) | 13.7 (8.3, 17.3) | 13.3 (8.0, 16.0) | 0.039 |
| Leukocytes (109/L) | 8.1 (3.8, 19.5) | 8.0 (5.1, 23.7) | 0.525 |
| Thrombocytes (109/L) | 306 (185, 654) | 293 (197, 785) | 0.896 |
| C-reactive protein (mg/L) | 11 (0, 156) | 14 (1, 236) | 0.417 |
| Albumin (g/dL) | 4.1 (1.6, 5.3) | 3.9 (2.5, 5.0) | 0.450 |
| Calprotectin (mg/kg) | 3452 (8, 43998) | 4166 (84, 36076) | 0.185 |
| LCN-2 (ng/mL) | 145 (6, 931) | 196 (14, 4014) | 0.572 |
| Lichtiger score | 10 (5, 17) | 10 (6, 16) | 0.568 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blesl, A.; Borenich, A.; Gröchenig, H.P.; Novacek, G.; Primas, C.; Reinisch, W.; Kutschera, M.; Illiasch, C.; Hennlich, B.; Steiner, P.; et al. Factors Associated with Response to Systemic Corticosteroids in Active Ulcerative Colitis: Results from a Prospective, Multicenter Trial. J. Clin. Med. 2023, 12, 4853. https://doi.org/10.3390/jcm12144853
Blesl A, Borenich A, Gröchenig HP, Novacek G, Primas C, Reinisch W, Kutschera M, Illiasch C, Hennlich B, Steiner P, et al. Factors Associated with Response to Systemic Corticosteroids in Active Ulcerative Colitis: Results from a Prospective, Multicenter Trial. Journal of Clinical Medicine. 2023; 12(14):4853. https://doi.org/10.3390/jcm12144853
Chicago/Turabian StyleBlesl, Andreas, Andrea Borenich, Hans Peter Gröchenig, Gottfried Novacek, Christian Primas, Walter Reinisch, Maximilian Kutschera, Constanze Illiasch, Barbara Hennlich, Pius Steiner, and et al. 2023. "Factors Associated with Response to Systemic Corticosteroids in Active Ulcerative Colitis: Results from a Prospective, Multicenter Trial" Journal of Clinical Medicine 12, no. 14: 4853. https://doi.org/10.3390/jcm12144853
APA StyleBlesl, A., Borenich, A., Gröchenig, H. P., Novacek, G., Primas, C., Reinisch, W., Kutschera, M., Illiasch, C., Hennlich, B., Steiner, P., Koch, R., Tillinger, W., Haas, T., Reicht, G., Mayer, A., Ludwiczek, O., Miehsler, W., Steidl, K., Binder, L., ... Högenauer, C., on behalf of the Austrian IBD Study Group (ATISG). (2023). Factors Associated with Response to Systemic Corticosteroids in Active Ulcerative Colitis: Results from a Prospective, Multicenter Trial. Journal of Clinical Medicine, 12(14), 4853. https://doi.org/10.3390/jcm12144853