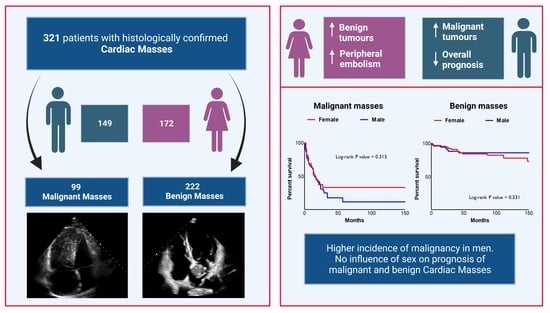
Sex-Related Disparities in Cardiac Masses: Clinical Features and Outcomes
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Data Collection and Follow Up
2.3. Echocardiographic Features in Women vs. Men
2.4. Statistical Analysis
3. Results
3.1. Female vs. Male: Clinical Features
3.2. Female vs. Males: Histological Subtypes
3.3. Female vs. Male: Echocardiographic Features
3.4. Outcomes according to Sex in Cardiac Masses
4. Discussion
4.1. Sex-Related Differences in Clinical Manifestations
4.2. Sex-Related Differences in Clinical Outcomes
4.3. Sex-Related Differences in Echocardiographic Features
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CCT | Cardiac Computed Tomography |
| CM | Cardiac Masses |
| CMR | Cardiac Magnetic Resonance |
| LVEF | Left Ventricular Ejection Fraction |
| WHO | World Health Organization |
| 18-FDG-PET | 18-Fluorodeoxyglucose Positron Emission Tomography |
References
- Amano, J.; Nakayama, J.; Yoshimura, Y.; Ikeda, U. Clinical classification of cardiovascular tumors and tumor-like lesions, and its incidences. Gen. Thorac. Cardiovasc. Surg. 2013, 61, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.; Tavora, F. The 2015 WHO Classification of Tumors of the Heart and Pericardium. J. Thorac. Oncol. 2016, 11, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Antwi-Amoabeng, D.; Meghji, Z.; Thakkar, S.; Ulanja, M.B.; Taha, M.; Adalja, D.; Al-Khafaji, J.; Gullapalli, N.; Beutler, B.D.; Boampong-Konam, K.; et al. Survival Differences in Men and Women with Primary Malignant Cardiac Tumor: An Analysis Using the Surveillance, Epidemiology and End Results (SEER) Database from 1973 to 2015. J. Am. Heart Assoc. 2020, 9, e014846. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Rizzo, S.; Valente, M.; Thiene, G. Cardiac masses and tumours. Heart 2016, 102, 1230–1245. [Google Scholar] [CrossRef]
- Miller, D.V.; Tazelaar, H.D. Cardiovascular Pseudoneoplasms. Arch. Pathol. Lab. Med. 2010, 134, 362–368. [Google Scholar] [CrossRef]
- Foà, A.; Paolisso, P.; Bergamaschi, L.; Rucci, P.; Di Marco, L.; Pacini, D.; Leone, O.; Galié, N.; Pizzi, C. Clues and pitfalls in the diagnostic approach to cardiac masses: Are pseudo-tumours truly benign? Eur. J. Prev. Cardiol. 2022, 29, e102–e104. [Google Scholar] [CrossRef]
- Bartoli, L.; Angeli, F.; Stefanizzi, A.; Fabrizio, M.; Paolisso, P.; Bergamaschi, L.; Broccoli, A.; Zinzani, P.L.; Galiè, N.; Rucci, P.; et al. Genetics and clinical phenotype of Erdheim–Chester disease: A case report of constrictive pericarditis and a systematic review of the literature. Front. Cardiovasc. Med. 2022, 9, 876294. [Google Scholar] [CrossRef]
- Bruce, C.J. Cardiac tumours: Diagnosis and management. Heart 2011, 97, 151–160. [Google Scholar] [CrossRef]
- D’angelo, E.C.; Paolisso, P.; Vitale, G.; Foà, A.; Bergamaschi, L.; Magnani, I.; Saturi, G.; Rinaldi, A.; Toniolo, S.; Renzulli, M.; et al. Diagnostic Accuracy of Cardiac Computed Tomography and 18-F Fluorodeoxyglucose Positron Emission Tomography in Cardiac Masses. JACC: Cardiovasc. Imaging 2020, 13, 2400–2411. [Google Scholar] [CrossRef]
- Lopes-Ramos, C.M.; Quackenbush, J.; DeMeo, D.L. Genome-Wide Sex and Gender Differences in Cancer. Front. Oncol. 2020, 10, 597788. [Google Scholar] [CrossRef]
- Dorak, M.T.; Karpuzoglu, E. Gender differences in cancer susceptibility: An inadequately addressed issue. Front. Genet. 2012, 3, 268. [Google Scholar] [CrossRef] [PubMed]
- Beery, T.A. Gender bias in the diagnosis and treatment of coronary artery disease. Heart Lung 1995, 24, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-I.; Lim, H.; Moon, A. Sex Differences in Cancer: Epidemiology, Genetics and Therapy. Biomol. Ther. 2018, 26, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Maron, M.S.; Adabag, A.S.; Casey, S.A.; Vargiu, D.; Link, M.S.; Udelson, J.E.; Cecchi, F.; Maron, B.J. Gender-Related Differences in the Clinical Presentation and Outcome of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2005, 46, 480–487. [Google Scholar] [CrossRef]
- Matthews, S.; Buttery, A.; O’neil, A.; Sanders, J.; Marasco, S.; Fredericks, S.; Martorella, G.; Keenan, N.; Ghanes, A.; Wynne, R. Sex differences in mortality after first time, isolated coronary artery bypass graft surgery: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Cardiovasc. Nurs. 2022, 21, 759–771. [Google Scholar] [CrossRef]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G., Jr.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed Modifications to the Duke Criteria for the Diagnosis of Infective Endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2018, 32, 1–64. [Google Scholar] [CrossRef]
- Paolisso, P.; Foà, A.; Bergamaschi, L.; Graziosi, M.; Rinaldi, A.; Magnani, I.; Angeli, F.; Stefanizzi, A.; Armillotta, M.; Sansonetti, A.; et al. Echocardiographic Markers in the Diagnosis of Cardiac Masses. J. Am. Soc. Echocardiogr. 2023. ahead of print. [Google Scholar] [CrossRef]
- Paolisso, P.; Foà, A.; Magnani, I.; Bergamaschi, L.; Graziosi, M.; Angeli, F.; Chiti, C.; Fabrizio, M.; Rinaldi, A.; Stefanizzi, A.; et al. Development and Validation of a Diagnostic Echocardiographic Mass Score in the Approach to Cardiac Masses. JACC: Cardiovasc. Imaging 2022, 15, 2010–2012. [Google Scholar] [CrossRef]
- Angeli, F.; Fabrizio, M.; Paolisso, P.; Magnani, I.; Bergamaschi, L.; Bartoli, L.; Stefanizzi, A.; Armillotta, M.; Sansonetti, A.; Amicone, S.; et al. [Cardiac masses: Classification, clinical features and diagnostic approach]. G Ital. Cardiol. 2022, 23, 620–630. [Google Scholar]
- Bergamaschi, L.; Foà, A.; Pizzi, C. Malignant Anterior ST-Segment Elevation. JAMA Intern. Med. 2022, 182, 672–673. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.K.M.M. Cardiac myxomas: A narrative review. World J. Cardiol. 2022, 14, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Singhal, P.; Luk, A.; Rao, V.; Butany, J. Molecular Basis of Cardiac Myxomas. Int. J. Mol. Sci. 2014, 15, 1315–1337. [Google Scholar] [CrossRef]
- AlAhmadi, H.H.; Alsafwani, N.S.; Shawarby, M.A.; Ahmed, F. Cardiac Myxoma: Typical Presentation but Unusual Histology. Case Rep. Med. 2021, 2021, 6611579. [Google Scholar] [CrossRef]
- Rahouma, M.; Baudo, M.; Shmushkevich, S.; Chadow, D.; Mohamed, A.; Girardi, L.; Gaudino, M.; Lorusso, R. Sex differences in primary malignant cardiac tumors: A multi-institutional cohort study from National Cancer Database. J. Card Surg. 2022, 37, 1275–1286. [Google Scholar] [CrossRef]
- Bussani, R.; Castrichini, M.; Restivo, L.; Fabris, E.; Porcari, A.; Ferro, F.; Pivetta, A.; Korcova, R.; Cappelletto, C.; Manca, P.; et al. Cardiac Tumors: Diagnosis, Prognosis, and Treatment. Curr. Cardiol. Rep. 2020, 22, 169. [Google Scholar] [CrossRef]
- López-Fernández, T.; Couch, L.S.; Aznar, M.C.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; Cutter, D.J.; de Azambuja, E.; de Boer, R.A.; et al. ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar]
- Cordioli, E.; Pizzi, C.; Bugiardini, R. Left ventricular metastasis from uterine leiomyosarcoma. Cardiologia 1999, 44, 1001–1003. [Google Scholar]


| Total Population N = 321 | Female Patients N = 172 | Male Patients N = 149 | p-Value | |
|---|---|---|---|---|
| Age in years, mean ± SD | 59.8 ± 15.4 | 61.4 ±15.7 | 57.9 ± 15.4 | 0.02 |
| BMI in kg/m2, mean ± SD | 25.4 ± 4.4 | 24.8 ± 4.1 | 26.3 ± 3.8 | 0.01 |
| Cardiovascular risk factors | ||||
| Smoking habit, n (%) | 160 (49.8) | 60 (34.8) | 100 (66.9) | <0.001 |
| Hypertension, n (%) | 180 (56.1) | 97 (56.4) | 83 (55.7) | 0.84 |
| Dyslipidemia, n (%) | 140 (43.6) | 81 (47) | 59 (39.9) | 0.22 |
| DM, n (%) | 48 (14.9) | 24 (13.9) | 24 (16.2) | 0.27 |
| Medical History | ||||
| Congestive Heart failure, n (%) | 40 (12.5) | 15 (8.7) | 25(16.8) | 0.06 |
| Prior stroke, n (%) | 70 (21.8) | 32 (18.6) | 28 (18.8) | 0.12 |
| Vasculopathy, n (%) | 78 (24.8) | 42 (24.4) | 36 (24.2) | 0.19 |
| History of neoplasia, n (%) | 0.53 | |||
| None | 210 (65.4) | 116 (67.4) | 94 (63.1) | |
| Benign | 32 (9.9) | 21 (12.2) | 11 (7.4) | |
| Malignant | 79 (24.7) | 41 (23.8) | 38 (25.5) | |
| CHA2DS2-VASc, mean ± SD | 2.6 ± 1.7 | 2.7 ± 1.6 | 2.4 ± 1.7 | 0.06 |
| Total Population N = 321 | Female Patients N = 172 | Male Patients N = 149 | p-Value | |
|---|---|---|---|---|
| Clinical features | ||||
| Malignant lesion, n (%) | 99 (30.8) | 35 (20.3) | 64 (43) | <0.001 |
| Accidental diagnosis, n (%) | 137 (42.7) | 71 (41.3) | 66 (44.3) | 0.56 |
| Dyspnea, n (%) | 129 (40.2) | 73 (42.4) | 56 (37.6) | 0.36 |
| NYHA Classification, n (%) | 0.26 | |||
| Class I–II | 244 (76) | 132 (76.8) | 112 (75.2) | |
| Class III–IV | 77 (24) | 40 (23.2) | 37 (24.8) | |
| Chest pain, n (%) | 52 (16.2) | 30 (17.4) | 22 (14.8) | 0.49 |
| Peripheral embolism, n (%) | 43 (13.4) | 32 (18.6) | 11 (7.4) | 0.003 |
| Pulmonary embolism, n (%) | 44 (13.7) | 24 (13.9) | 20 (13.4) | 0.1 |
| Location, n (%) | 0.087 | |||
| Right cardiac chambers, n (%) | 93 (29) | 45 (26.2) | 48 (32.2) | |
| Left cardiac chambers, n (%) | 174 (54.2) | 104 (60.5) | 70 (47) | |
| Pericardium, n (%) | 36 (11.2) | 15 (8.7) | 21 (14) | |
| Great Vessels, n (%) | 18 (5.6) | 8 (4.6) | 10 (6.8) | |
| Laboratory parameters | ||||
| Creatinine in mg/dL, mean ± SD | 1.04 ± 0.67 | 0.88 ± 0.65 | 1.22 ±0.8 | 0.002 |
| GFR in mL/min, mean ± SD | 80.4 ±26.3 | 84.3 ± 23.1 | 75.8 ±28.9 | 0.003 |
| Hb in g/dL, mean ± SD | 12.7 ± 2 | 12.4 ± 1.6 | 13.2 ± 2.1 | 0.001 |
| WBC in n/mmc, mean ± SD | 8522 ± 3846 | 8472 ±3121 | 8579 ±4114 | 0.36 |
| LDH in U/L, median [IQR] | 231 [155–289] | 222 [143–272] | 244 [135–279] | 0.67 |
| CRP in mg/dL, median [IQR] | 1.89 [3.5–0.45] | 1.5 [3.2–0.3] | 1.7 [3.4–0.7] | 0.07 |
| Total Population N = 321 | Female Patients N = 172 | Male Patients N = 149 | p-Value | |
|---|---|---|---|---|
| Benign tumours | 149 (46.4) | 101 (58.7) | 48 (32.2) | <0.001 |
| Myxoma | 114 (76.5) | 82 (81.2) | 32 (64.6) | |
| Fibroelastoma | 23 (15.4) | 12 (11.2) | 11 (22.9) | |
| Lipoma | 5 (3.2) | 2 (2) | 3 (6.3) | |
| Fibroma | 3 (2) | 2 (2) | 1 (2) | |
| Paraganglioma | 3 (2) | 2 (2) | 1 (2) | |
| Hamartoma | 1 (0.7) | 1 (0.9) | 0 (0) | |
| Pseudotumours | 73 (22.7) | 36 (20.1) | 37 (24.8) | 0.43 |
| Thrombus | 37 (50.7) | 20 (55.56) | 17 (45.9) | |
| Cyst | 10 (13.7) | 5 (13.9) | 5 (13.5) | |
| Valvular nodule | 10 (13.7) | 3 (8.3) | 7 (18.9) | |
| Lipomatosis | 6 (8.3) | 4 (11.1) | 2 (5.4) | |
| Reactive inflammatory process | 6 (8.3) | 3 (8.3) | 3 (8.1) | |
| Calcification | 3 (4.1) | 0 (0) | 3 (8.1) | |
| Cystic atrioventricular node tumour | 1 (1.7) | 1 (2.8) | 0 (0) | |
| Primary malignant tumours | 35 (10.9) | 15 (8.7) | 20 (13.4) | 0.21 |
| Sarcoma | 31 (88.6) | 14 (93.3) | 17 (85) | |
| Lymphoma | 3 (8.6) | 0 (0) | 3 (15) | |
| Mesothelioma | 1 (2.8) | 1 (6.7) | 0 (0) | |
| Metastatic tumours | 64 (19.9) | 20 (11.6) | 44 (29.5) | <0.001 |
| Lymphoma | 23 (35.9) | 5 (25) | 18 (40.9) | |
| Lung carcinoma | 8 (12.5) | 2 (10) | 6 (13.6) | |
| Sarcoma | 7 (10.9) | 2 (10) | 5 (11.4) | |
| Renal and urological tumours | 7 (10.9) | 3 (15) | 4 (9.1) | |
| Melanoma | 6 (9.3) | 2 (10) | 4 (9.1) | |
| Hepatocellular carcinoma | 5 (7.8) | 1 (5) | 4 (9.1) | |
| Colon carcinoma | 4 (6.3) | 1 (5) | 3 (6.8) | |
| Gynaecological tumours | 3 (4.7) | 3 (15) | 0 | |
| Plasmacytoma | 1 (1.6) | 1 (5) | 0 |
| Total Population N = 286 | Female Patients N = 155 | Male Patients N = 131 | p-Value | |
|---|---|---|---|---|
| Ventricular parameters | ||||
| Ejection Fraction in %, mean ± SD | 60.4 ± 9.7 | 62.3 ±8.3 | 58.4 ± 11.2 | 0.001 |
| LV EDD in mm, mean ± SD | 46.7 ±6.4 | 45 ± 5.7 | 48.7 ± 6.6 | <0.001 |
| LV EDV in mm3, mean ± SD | 92.9 ± 35.1 | 81 ± 24.8 | 107.2 ± 40.9 | <0.001 |
| LAV in mL, mean ± SD | 51 ± 5 | 48 ± 3 | 55 ± 4 | 0.067 |
| DDF, n (%) | 13 (4.5) | 7 (4.6) | 6 (4.5) | 0.98 |
| sysPAP in mmHg, mean ± SD | 34.9 ± 15 | 35.8 ± 18 | 32.8 ± 12.3 | 0.79 |
| Mitral regurgitation, n (%) | 0.87 | |||
| Mild | 160 (55.9) | 91 (58.7) | 69 (52.7) | |
| Moderate/severe | 40 (14) | 20 (12.9) | 20 (15.3) | |
| Mitral stenosis, n (%) | 0.2 | |||
| Mild | 8 (2.8) | 6 (3.9) | 2 (1.5) | |
| Moderate/severe | 12 (4.2) | 8 (5.2) | 4 (3.1) | |
| Aortic regurgitation, n (%) | 0.56 | |||
| Mild | 61 (21.3) | 36 (23.2) | 25 (19.1) | |
| Moderate/severe | 13 (4.5) | 8 (5.2) | 5 (3.8) | |
| Aortic stenosis, n (%) | 0.6 | |||
| Mild | 22 (7.7) | 11 (7.1) | 11 (8.4) | |
| Moderate/severe | 5 (1.7) | 4 (2.6) | 1 (0.8) | |
| Tricuspid regurgitation, n (%) | 0.54 | |||
| Mild | 159 (55.6) | 88 (56.8) | 71 (54.2) | |
| Moderate/severe | 26 (9.1) | 10 (7.6) | 16 (10.3) | |
| Pericardial effusion, n (%) | 63 (22) | 21 (13.5) | 42 (32.1) | 0.001 |
| Mild, n (%) | 34 (53.9) | 15 (71.4) | 19 (45.2) | |
| Moderate/severe, n (%) | 29 (46.1) | 6 (28.6) | 23 (54.7) | |
| Mass features | ||||
| Infiltration, n (%) | 57 (19.9) | 19 (12.3) | 38 (29) | 0.001 |
| Max CM diameter in mm, mean ± SD | 36 ±19.6 | 33.8 ±15.9 | 38.6 ±22.9 | 0.02 |
| Inhomogeneity, n (%) | 73 (25.5) | 35 (22.6) | 38 (29) | 0.22 |
| Irregular margins, n (%) | 72 (25.2) | 24 (15.5) | 48 (36.6) | <0.001 |
| Mobility, n (%) | 143 (50) | 88 (56.8) | 55 (42) | 0.04 |
| Sessile mass, n (%) | 129 (45.1) | 62 (40) | 67 (51.1) | 0.02 |
| Polylobate mass, n (%) | 72 (25.2) | 29 (18.7) | 43 (32.8) | 0.003 |
| Total Population | Female Patients | Male Patients | p-Value | |
|---|---|---|---|---|
| Benign tumours | 17/149 (11.4) | 14/101 (13.9) | 3/48 (6.3) | 0.27 |
| Primary Malignant tumours | 23/35 (65.7) | 8/15 (53.3) | 15/20 (75.5) | 0.12 |
| Pseudotumours | 10/73 (13.7) | 5/36 (13.9) | 5/37 (13.5) | 0.62 |
| Metastatic tumours | 40/64 (63) | 13/20 (65) | 27/44 (61.4) | 0.51 |
| Univariate Analysis | Multivariate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | Standard Error | 95% CI | p Value | Odds Ratio | Standard Error | 95% CI | p Value | |
| Age | 1.02 | 0.008 | 1.04–1.034 | 0.01 | 1.03 | 0.08 | 1.04–1.01 | <0.001 |
| Male Sex | 1.76 | 0.21 | 2.66–1.157 | 0.008 | - | - | - | - |
| Smoking | 1.38 | 0.21 | 2.09–0.91 | 0.132 | 1.55 | 0.22 | 2.35–1.001 | 0.049 |
| Hypertension | 0.81 | 0.22 | 1.24–0.5 | 0.33 | - | - | - | - |
| Dyslipidaemia | 1.11 | 0.21 | 1.69–0.73 | 0.62 | - | - | - | - |
| Diabetes Mellitus | 0.75 | 0.28 | 1.29–0.44 | 0.3 | - | - | - | - |
| Peripheral artery disease | 1.22 | 0.24 | 1.95–0.76 | 0.41 | - | - | - | - |
| Malignant tumour | 9.98 | 0.24 | 15.9–6.26 | <0.001 | 13.32 | 0.26 | 21.98–8.07 | <0.001 |
| Peripheral embolism | 0.97 | 0.3 | 1.74–0.54 | 0.914 | 1.94 | 0.32 | 3.61–1.05 | 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angeli, F.; Bergamaschi, L.; Rinaldi, A.; Paolisso, P.; Armillotta, M.; Stefanizzi, A.; Sansonetti, A.; Amicone, S.; Impellizzeri, A.; Bodega, F.; et al. Sex-Related Disparities in Cardiac Masses: Clinical Features and Outcomes. J. Clin. Med. 2023, 12, 2958. https://doi.org/10.3390/jcm12082958
Angeli F, Bergamaschi L, Rinaldi A, Paolisso P, Armillotta M, Stefanizzi A, Sansonetti A, Amicone S, Impellizzeri A, Bodega F, et al. Sex-Related Disparities in Cardiac Masses: Clinical Features and Outcomes. Journal of Clinical Medicine. 2023; 12(8):2958. https://doi.org/10.3390/jcm12082958
Chicago/Turabian StyleAngeli, Francesco, Luca Bergamaschi, Andrea Rinaldi, Pasquale Paolisso, Matteo Armillotta, Andrea Stefanizzi, Angelo Sansonetti, Sara Amicone, Andrea Impellizzeri, Francesca Bodega, and et al. 2023. "Sex-Related Disparities in Cardiac Masses: Clinical Features and Outcomes" Journal of Clinical Medicine 12, no. 8: 2958. https://doi.org/10.3390/jcm12082958
APA StyleAngeli, F., Bergamaschi, L., Rinaldi, A., Paolisso, P., Armillotta, M., Stefanizzi, A., Sansonetti, A., Amicone, S., Impellizzeri, A., Bodega, F., Canton, L., Suma, N., Fedele, D., Bertolini, D., Tattilo, F. P., Cavallo, D., Di Iuorio, O., Ryabenko, K., Casuso Alvarez, M., ... Pizzi, C. (2023). Sex-Related Disparities in Cardiac Masses: Clinical Features and Outcomes. Journal of Clinical Medicine, 12(8), 2958. https://doi.org/10.3390/jcm12082958