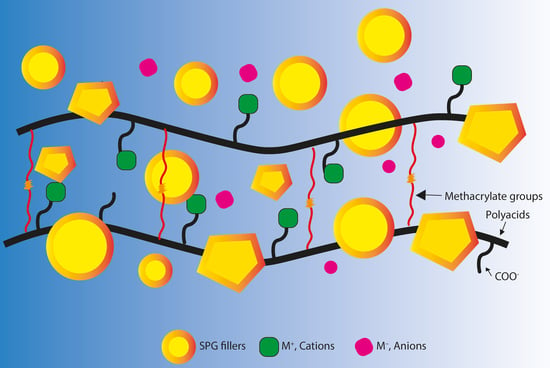
Rheological Properties, Surface Microhardness, and Dentin Shear Bond Strength of Resin-Modified Glass Ionomer Cements Containing Methacrylate-Functionalized Polyacids and Spherical Pre-Reacted Glass Fillers
Abstract
:1. Introduction
2. Material and Methods
2.1. Material Preparation
2.2. Rheological Test
2.3. Vickers Surface Microhardness Testing
2.4. Shear Bond Strength
- (1)
- Adhesive failure between material and dentin.
- (2)
- Cohesive failure mode within material.
- (3)
- Mixed failure mode with both cohesive and adhesive failure.
2.5. Statistical Analysis
3. Results
3.1. FTIR Studies
3.2. Rheological Properties
3.3. Vickers Surface Microhardness
3.4. Shear Bond Strength (SBS)
4. Discussion
4.1. FTIR Studies
4.2. Rheological Properties
4.3. Surface Microhardness
4.4. Shear Bond Strength
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD Oral Disorders Collaborators; Bernabé, E.; Marcenes, W.; Hernandez, C.R.; Bailey, J.; Abreu, L.G.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z.; et al. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Duncan, H.; Galler, K.M.; Tomson, P.L.; Simon, S.; El Karim, I.; Kundzina, R.; Krastl, G.; Dammaschke, T.; Fransson, H.; Markvart, M.; et al. European Society of Endodontology position statement: Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 923–934. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.; Frencken, J.E.; Schwendicke, F.; Innes, N.P.T. Contemporary operative caries management: Consensus recommendations on minimally invasive caries removal. Br. Dent. J. 2017, 223, 215–222. [Google Scholar] [CrossRef]
- Schwendicke, F. Contemporary concepts in carious tissue removal: A review. J. Esthet. Restor. Dent. 2017, 29, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mittal, S.; Tewari, S. Effect of Different Liners on Pulpal Outcome after Partial Caries Removal: A Preliminary 12 Months Randomised Controlled Trial. Caries Res. 2019, 53, 547–554. [Google Scholar] [CrossRef]
- Chai, B.Y.Y.; Tay, B.Y.X.; Chow, C.Y.T.; Fuss, J.; Krishnan, U. Treatment preferences for deep caries lesions among Australian dentists. Aust. Dent. J. 2019, 65, 83–89. [Google Scholar] [CrossRef]
- Aggarwal, V.; Singla, M.; Yadav, S.; Yadav, H. Effect of flowable composite liner and glass ionomer liner on class II gingival marginal adaptation of direct composite restorations with different bonding strategies. J. Dent. 2014, 42, 619–625. [Google Scholar] [CrossRef]
- Kazemipoor, M.; Azizi, N.; Farahat, F. Evaluation of Microhardness of Mineral Trioxide Aggregate After Immediate Placement of Different Coronal Restorations: An In Vitro Study. J. Dent. 2018, 15, 116–122. [Google Scholar]
- Karadas, M.; Atıcı, M.G. Bond strength and adaptation of pulp capping materials to dentin. Microsc. Res. Tech. 2020, 83, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Khokhar, M.; Tewari, S. Outcomes of Partial and Complete Caries Excavation in Permanent Teeth: A 18 Month Clinical Study. Contemp. Clin. Dent. 2018, 9, 468–473. [Google Scholar] [CrossRef]
- Sidhu, S.K.; Nicholson, J.W. A Review of Glass-Ionomer Cements for Clinical Dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef]
- Ugurlu, M. Bonding of a resin-modified glass ionomer cement to dentin using universal adhesives. Restor. Dent. Endod. 2020, 45, e36. [Google Scholar] [CrossRef]
- Jochums, A.; Volk, J.; Perduns, R.; Plum, M.; Schertl, P.; Bakopoulou, A.; Geurtsen, W. Influence of 2-hydroxyethyl methacrylate (HEMA) exposure on angiogenic differentiation of dental pulp stem cells (DPSCs). Dent. Mater. 2021, 37, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Baldion, P.A.; Velandia-Romero, M.L.; Castellanos, J.E. Dental resin monomers induce early and potent oxidative damage on human odontoblast-like cells. Chem. Interact. 2021, 333, 109336. [Google Scholar] [CrossRef]
- Massaro, H.; Zambelli, L.F.A.; De Britto, A.A.; Vieira, R.P.; Ligeiro-De-Oliveira, A.P.; Andia, D.C.; Oliveira, M.T.; Lima, A.F. Solvent and HEMA Increase Adhesive Toxicity and Cytokine Release from Dental Pulp Cells. Materials 2019, 12, 2750. [Google Scholar] [CrossRef] [Green Version]
- Mulligan, S.; Kakonyi, G.; Moharamzadeh, K.; Thornton, S.F.; Martin, N. The environmental impact of dental amalgam and resin-based composite materials. Br. Dent. J. 2018, 224, 542–548. [Google Scholar] [CrossRef]
- Channasanon, S.; Soodsawang, W.; Monmaturapoj, N.; Tanodekaew, S. Improving flexural strength of resin modified glass-ionomer cement by poly (alkenoic acid) modifications. Songklanakarin J. Sci. Technol. 2013, 35, 429–436. [Google Scholar]
- Xie, D.; Park, J.-G.; Zhao, J. Synthesis and preparation of novel 4-arm star-shape poly(carboxylic acid)s for improved light-cured glass-ionomer cements. Dent. Mater. 2007, 23, 395–403. [Google Scholar] [CrossRef]
- Xie, D.; Chung, I.-D.; Wu, W.; Mays, J. Synthesis and evaluation of HEMA-free glass–ionomer cements for dental applications. Dent. Mater. 2004, 20, 470–478. [Google Scholar] [CrossRef]
- Zanchi, C.H.; Münchow, E.A.; Ogliari, F.A.; De Carvalho, R.V.; Chersoni, S.; Prati, C.; Demarco, F.F.; Piva, E. A new approach in self-etching adhesive formulations: Replacing HEMA for surfactant dimethacrylate monomers. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 99, 51–57. [Google Scholar] [CrossRef]
- Panpisut, P.; Monmaturapoj, N.; Srion, A.; Toneluck, A.; Phantumvanit, P. Physical Properties of Glass Ionomer Cement Containing Pre-Reacted Spherical Glass Fillers. Braz. Dent. J. 2020, 31, 445–452. [Google Scholar] [CrossRef]
- Panpisut, P.; Monmaturapoj, N.; Srion, A.; Angkananuwat, C.; Krajangta, N.; Panthumvanit, P. The effect of powder to liquid ratio on physical properties and fluoride release of glass ionomer cements containing pre-reacted spherical glass fillers. Dent. Mater. J. 2020, 39, 563–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monmaturapoj, N.; Soodsawang, W.; Tanodekaew, S. Enhancement effect of pre-reacted glass on strength of glass-ionomer cement. Dent. Mater. J. 2012, 31, 125–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tcharkhtchi, A.; Nony, F.; Khelladi, S.; Fitoussi, J.; Farzaneh, S. Epoxy/amine reactive systems for composites materials and their thermomechanical properties. In Advances in Composites Manufacturing and Process Design; Boisse, P., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 269–296. [Google Scholar]
- Mori, T.; Takase, K.; Yoshida, K.; Okazaki, H.; Murata, H. Influence of monomer type, plasticizer content, and powder/liquid ratio on setting characteristics of acrylic permanent soft denture liners based on poly(ethyl methacrylate/butyl methacrylate) and acetyl tributyl citrate. Dent. Mater. J. 2021, 2020–2319. [Google Scholar] [CrossRef]
- Tsuka, H.; Morita, K.; Kato, K.; Kawano, H.; Abekura, H.; Tsuga, K. Evaluation of shear bond strength between PEEK and resin-based luting material. J. Oral Biosci. 2017, 59, 231–236. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Young, A.; Rafeeka, S.; Howlett, J. FTIR investigation of monomer polymerisation and polyacid neutralisation kinetics and mechanisms in various aesthetic dental restorative materials. Biomaterials 2004, 25, 823–833. [Google Scholar] [CrossRef]
- Delgado, A.H.S.; Young, A.M. Methacrylate peak determination and selection recommendations using ATR-FTIR to investigate polymerisation of dental methacrylate mixtures. PLoS ONE 2021, 16, e0252999. [Google Scholar] [CrossRef]
- Abraham, S.B.; Gaintantzopoulou, M.D.; Eliades, G. Cavity Adaptation of Water-Based Restoratives Placed as Liners under a Resin Composite. Int. J. Dent. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Xie, D.; Chung, I.-D.; Wu, W.; Lemons, J.; Puckett, A.; Mays, J. An amino acid-modified and non-HEMA containing glass-ionomer cement. Biomaterials 2004, 25, 1825–1830. [Google Scholar] [CrossRef]
- Dörr, D.; Kuhn, U.; Altstädt, V. Rheological Study of Gelation and Crosslinking in Chemical Modified Polyamide 12 Using a Multiwave Technique. Polymers 2020, 12, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, B.S.; Chandler, N. Root Canal Filling Materials and Techniques. In Endodontic Materials in Clinical Practice; John Wiley & Sons Ltd.: Oxford, UK, 2021; pp. 181–217. [Google Scholar]
- De Caluwé, T.; Vercruysse, C.; Fraeyman, S.; Verbeeck, R. The influence of particle size and fluorine content of aluminosilicate glass on the glass ionomer cement properties. Dent. Mater. 2014, 30, 1029–1038. [Google Scholar] [CrossRef]
- Bonsor, S.J. Contemporary strategies and materials to protect the dental pulp. Dent. Update 2017, 44, 731–741. [Google Scholar] [CrossRef]
- Moheet, I.; Luddin, N.; Ab Rahman, I.; Masudi, S.M.; Kannan, T.P.; Ghani, N.R.N.A. Evaluation of mechanical properties and bond strength of nano-hydroxyapatite-silica added glass ionomer cement. Ceram. Int. 2018, 44, 9899–9906. [Google Scholar] [CrossRef]
- Nicholson, J.W.; Sidhu, S.K.; Czarnecka, B. Enhancing the Mechanical Properties of Glass-Ionomer Dental Cements: A Review. Materials 2020, 13, 2510. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chiu, Y.-C.; Shen, Y.-F.; Wu, Y.-H.A.; Shie, M.-Y. Bioactive calcium silicate/poly-ε-caprolactone composite scaffolds 3D printed under mild conditions for bone tissue engineering. J. Mater. Sci. Mater. Med. 2017, 29, 11. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Wetzel, R.; Eckardt, O.; Biehl, P.; Brauer, D.; Schacher, F. Effect of poly(acrylic acid) architecture on setting and mechanical properties of glass ionomer cements. Dent. Mater. 2020, 36, 377–386. [Google Scholar] [CrossRef]
- Kundie, F.; Azhari, C.H.; Muchtar, A.; Ahmad, Z.A. Effects of Filler Size on the Mechanical Properties of Polymer-filled Dental Composites: A Review of Recent Developments. J. Phys. Sci. 2018, 29, 141–165. [Google Scholar] [CrossRef] [Green Version]
- Meraji, N.; Nekoofar, M.H.; Yazdi, K.A.; Sharifian, M.R.; Fakhari, N.; Camilleri, J. Bonding to caries affected dentine. Dent. Mater. 2018, 34, e236–e245. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Yoshihara, K.; Van Landuyt, K.; Yoshida, Y.; Peumans, M. From Buonocore’s Pioneering Acid-Etch Technique to Self-Adhering Restoratives. A Status Perspective of Rapidly Advancing Dental Adhesive Technology. J. Adhes. Dent. 2020, 22, 7–34. [Google Scholar] [CrossRef]
- Saad, A.; Inoue, G.; Nikaido, T.; Ikeda, M.; Burrow, M.; Tagami, J. Microtensile Bond Strength of Resin-Modified Glass Ionomer Cement to Sound and Artificial Caries–Affected Root Dentin with Different Conditioning. Oper. Dent. 2017, 42, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Poorzandpoush, K.; Shahrabi, M.; Heidari, A.; Hosseinipour, Z.S. Shear Bond Strength of Self-Adhesive Flowable Composite, Conventional Flowable Composite and Resin-Modified Glass Ionomer Cement to Primary Dentin. Front. Dent. 2019, 16, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, A.; Abrar, E.; Maawadh, A.M.; Al-Hamdan, R.S.; Almohareb, T.; AlFawaz, Y.; Naseem, M.; Vohra, F.; Abduljabbar, T. Management of caries affected dentin (CAD) with resin modified glass ionomer cement (RMGIC) in the presence of different caries disinfectants and photosensitizers. Photodiagn. Photodyn. Ther. 2020, 32, 101978. [Google Scholar] [CrossRef]
- Zhao, I.S.; Mei, M.L.; Zhou, Z.L.; Burrow, M.F.; Lo, E.C.-M.; Chu, C.-H. Shear Bond Strength and Remineralisation Effect of a Casein Phosphopeptide-Amorphous Calcium Phosphate-Modified Glass Ionomer Cement on Artificial “Caries-Affected” Dentine. Int. J. Mol. Sci. 2017, 18, 1723. [Google Scholar] [CrossRef] [Green Version]
- Al-Khureif, A.A.; Mohamed, B.A.; Al-Shehri, A.M.; Khan, A.A.; Divakar, D.D. Bond assessment of resin modified glass ionomer cement to dentin conditioned with photosensitizers, laser and conventional regimes. Photodiagn. Photodyn. Ther. 2020, 30, 101795. [Google Scholar] [CrossRef]
- El Mourad, A.M. Assessment of Bonding Effectiveness of Adhesive Materials to Tooth Structure using Bond Strength Test Methods: A Review of Literature. Open Dent. J. 2018, 12, 664–678. [Google Scholar] [CrossRef] [Green Version]
- Sirisha, K.; Rambabu, T.; Shankar, Y.R.; Ravikumar, P. Validity of bond strength tests: A critical review: Part I. J. Conserv. Dent. 2014, 17, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Saad, A.; Inoue, G.; Nikaido, T.; Abdou, A.; Sayed, M.; Burrow, M.F.; Tagami, J. Effect of dentin contamination with two hemostatic agents on bond strength of resin-modified glass ionomer cement with different conditioning. Dent. Mater. J. 2019, 38, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Hoshika, S.; De Munck, J.; Sano, H.; Sidhu, S.K.; Van Meerbeek, B. Effect of Conditioning and Aging on the Bond Strength and Interfacial Morphology of Glass-ionomer Cement Bonded to Dentin. J. Adhes. Dent. 2015, 17, 141–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshihara, K.; Nagaoka, N.; Okihara, T.; Irie, M.; Matsukawa, A.; Pedano, M.S.; Maruo, Y.; Yoshida, Y.; Van Meerbeek, B. Development of self-adhesive pulp-capping agents containing a novel hydrophilic and highly polymerizable acrylamide monomer. J. Mater. Chem. B 2020, 8, 5320–5329. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Hong, M.-H.; Kwon, T.-Y. Dentin Bonding of TheraCal LC Calcium Silicate Containing an Acidic Monomer: An In Vitro Study. Materials 2020, 13, 293. [Google Scholar] [CrossRef] [Green Version]
- Cadenaro, M.; Maravic, T.; Comba, A.; Mazzoni, A.; Fanfoni, L.; Hilton, T.; Ferracane, J.; Breschi, L. The role of polymerization in adhesive dentistry. Dent. Mater. 2019, 35, e1–e22. [Google Scholar] [CrossRef]
- Aljabo, A.; Xia, W.; Liaqat, S.; Khan, M.; Knowles, J.; Ashley, P.; Young, A. Conversion, shrinkage, water sorption, flexural strength and modulus of re-mineralizing dental composites. Dent. Mater. 2015, 31, 1279–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Liquid Formulations | Composition |
|---|---|
| CM | CM polymer (55 wt. %), water (45 wt. %), tartaric acid (2 pph 1), camphorquinone (0.7 pph), N,N′-dimethylaminoethyl methacrylate (1.4 pph) |
| CMH | CM polymer (50 wt. %), water (45 wt. %), 2-hydroxyethyl methacrylate (5 wt. %), tartaric acid (2 pph), camphorquinone (0.7 pph), N,N′-dimethylaminoethyl methacrylate (1.4 pph) |
| Materials | Composition | Instruction | Suppliers | Lot No. |
|---|---|---|---|---|
| Vitrebond (VB) | Powder: glass powder (>95 wt. %), diphenyliodonium chloride (<2 wt. %) Liquid: copolymer of acrylic and itaconic acids (35–45 wt. %), 2-hydroxyethyl methacrylate (20–30 wt. %), water (30–40 wt. %) Powder-to-liquid ratio: 1.4:1 (mass ratio) |
| 3M ESPE, St. Paul, MN, USA | BN981834 |
| TheraCal LC (TC) | Calcium-silicate cement (30–50 wt. %), polyethylene glycol dimethacrylate (10–30 wt. %), barium zirconate powder (1–10 wt. %) |
| Bisco Inc., Schaumburg, IL, USA | 1900006662 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thepveera, W.; Potiprapanpong, W.; Toneluck, A.; Channasanon, S.; Khamsuk, C.; Monmaturapoj, N.; Tanodekaew, S.; Panpisut, P. Rheological Properties, Surface Microhardness, and Dentin Shear Bond Strength of Resin-Modified Glass Ionomer Cements Containing Methacrylate-Functionalized Polyacids and Spherical Pre-Reacted Glass Fillers. J. Funct. Biomater. 2021, 12, 42. https://doi.org/10.3390/jfb12030042
Thepveera W, Potiprapanpong W, Toneluck A, Channasanon S, Khamsuk C, Monmaturapoj N, Tanodekaew S, Panpisut P. Rheological Properties, Surface Microhardness, and Dentin Shear Bond Strength of Resin-Modified Glass Ionomer Cements Containing Methacrylate-Functionalized Polyacids and Spherical Pre-Reacted Glass Fillers. Journal of Functional Biomaterials. 2021; 12(3):42. https://doi.org/10.3390/jfb12030042
Chicago/Turabian StyleThepveera, Whithipa, Wisitsin Potiprapanpong, Arnit Toneluck, Somruethai Channasanon, Chutikarn Khamsuk, Naruporn Monmaturapoj, Siriporn Tanodekaew, and Piyaphong Panpisut. 2021. "Rheological Properties, Surface Microhardness, and Dentin Shear Bond Strength of Resin-Modified Glass Ionomer Cements Containing Methacrylate-Functionalized Polyacids and Spherical Pre-Reacted Glass Fillers" Journal of Functional Biomaterials 12, no. 3: 42. https://doi.org/10.3390/jfb12030042
APA StyleThepveera, W., Potiprapanpong, W., Toneluck, A., Channasanon, S., Khamsuk, C., Monmaturapoj, N., Tanodekaew, S., & Panpisut, P. (2021). Rheological Properties, Surface Microhardness, and Dentin Shear Bond Strength of Resin-Modified Glass Ionomer Cements Containing Methacrylate-Functionalized Polyacids and Spherical Pre-Reacted Glass Fillers. Journal of Functional Biomaterials, 12(3), 42. https://doi.org/10.3390/jfb12030042