TiO2 Nanoparticles Dispersion in Block-Copolymer Aqueous Solutions: Nanoarchitectonics for Self-Assembly and Aggregation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. UV-Vis Spectroscopy
2.4. DLS Measurements
2.5. FTIR-ATR Spectroscopy
2.6. Electrical Conductivity
3. Results and Discussion
3.1. UV-Vis Spectroscopy
3.2. FTIR-ATR Measurements
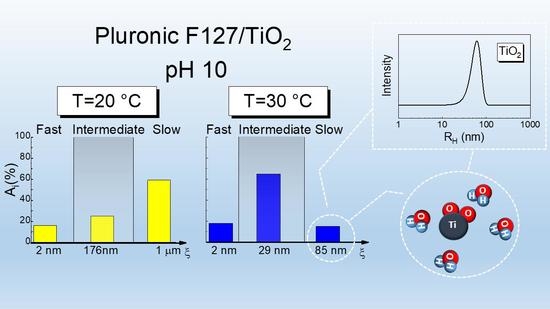
3.3. DLS Measurements
3.4. Electrical Conductivity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choi, H.; Sofranko, A.C.; Dionysiou, D.D. Nanocrystalline TiO2 photocatalytic membranes with a hierarchical mesoporous multilayer structure: Synthesis, characterization, and multifunction. Adv. Funct. Mater. 2006, 16, 1067–1074. [Google Scholar] [CrossRef]
- Chuangchote, S.; Jitputti, J.; Sagawa, T.; Yoshikawa, S. Photocatalytic activity for hydrogen evolution of electrospun TiO2 nanofibers. ACS Appl. Mater. Interfaces 2009, 1, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.; Grätzel, M. Low cost photovoltaic modules based on dye sensitized nanocrystalline titanium dioxide and carbon powder. Sol. Energy Mater. Sol. Cells 1996, 44, 99–117. [Google Scholar] [CrossRef]
- Arango, A.C.; Johnson, L.R.; Bliznyuk, V.N.; Schlesinger, Z.; Carter, S.A.; Hörhold, H.-H. Efficient titanium oxide/conjugated polymer photovoltaics for solar energy conversion. Adv. Mater. 2000, 12, 1689–1692. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Enhanced photocleavage of water using titania nanotube arrays. Nano Lett. 2005, 5, 191–195. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Turchi, C.S.; Ollis, D.F. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack. J. Catal. 1990, 122, 178–192. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Pelizzetti, E. Concluding remarks on heterogeneous solar photocatalysis. Sol. Energy Mater. Sol. Cells 1995, 38, 453–457. [Google Scholar] [CrossRef]
- Berardinelli, A.; Parisi, F. TiO2 in the food industry and cosmetics. In Metal Oxides, Titanium Dioxide (Tio2) and Its Applications; Parrino, F., Palmisano, L., Eds.; Elsevier: Hoboken, NJ, USA, 2021; pp. 353–371. [Google Scholar] [CrossRef]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012, 14, 1109–1120. [Google Scholar] [CrossRef] [Green Version]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Huang, J.-Y.; Li, H.-Q.; Chen, Z.; Zhao, A.Z.-J.; Wang, Y.; Zhang, K.-Q.; Sun, H.-T.; Al-Deyab, S.S.; Lai, Y.-K. TiO2 nanotube platforms for smart drug delivery: A review. Int. J. Nanomed. 2016, 11, 4819–4834. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Cimpean, A.; Tesler, A.B.; Mazare, A. Anodic TiO2 nanotubes: Tailoring Osteoinduction via Drug Delivery. Nanomaterials 2021, 11, 2359. [Google Scholar] [CrossRef]
- Babitha, S.; Korrapati, P.S. Biodegradable zein-polydopamine polymeric sca_old impregnated with TiO2 nanoparticles for skin tissue engineering. Biomed. Mater. 2017, 12, 055008. [Google Scholar] [CrossRef]
- Stan, M.S.; Nica, I.C.; Dinischiotu, A.; Varzaru, E.; Iordache, O.G.; Dumitrescu, I.; Popa, M.; Chifiriuc, M.C.; Pircalabioru, G.G.; Lazar, V.; et al. Photocatalytic, antimicrobial and biocompatibility features of cotton knitcoated with Fe-N-Doped titanium dioxide nanoparticles. Materials 2016, 9, 789. [Google Scholar] [CrossRef] [Green Version]
- Seisenbaeva, G.A.; Fromell, K.; Vinogradov, V.V.; Terekhov, A.N.; Pakhomov, A.V.; Nilsson, B.; Ekdahl, K.N.; Vinogradov, V.V.; Kessler, V.G. Dispersion of TiO2 nanoparticles improves burn wound healing and tissue regeneration through specific interaction with blood serum proteins. Sci. Rep. 2017, 7, 15448. [Google Scholar] [CrossRef] [Green Version]
- Hasan, K.M.F.; Horváth, P.G.; Alpár, T. Potential Natural Fiber Polymeric Nanobiocomposites: A Review. Polymers 2020, 12, 1072. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Almquist, C.B.; Biswas, P. Role of Synthesis Method and Particle Size of Nanostructured TiO2 on its Photoactivity. J. Catal. 2002, 212, 145–156. [Google Scholar] [CrossRef]
- Sclafani, A.; Herrmann, J.M. Comparison of the Photoelectronic and Photocatalytic Activities of various anatase and rutile forms of titania in pure liquid organic phases and in aqueous solutions. J. Phys. Chem. 1996, 100, 13655–13661. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Elder, A.; Gelein, R.; Mercer, P.; Biswas, P. Does nanoparticle activity depend upon size and crystal phase? Nanotoxicology 2008, 2, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Waychunas, G.A.; Kim, C.S.; Banfield, J.F. Nanoparticulate Iron Oxide Minerals in soils and sediments: Unique properties and contaminant scavenging mechanisms. J. Nanopart. Res. 2005, 7, 409–433. [Google Scholar] [CrossRef]
- Kaasalainen, M.; Aseyev, V.; von Haartman, E.; Şen Karaman, D.; Mäkilä, E.; Tenhu, H.; Rosenholm, J.; Salonen, J. Size, Stability, and Porosity of Mesoporous Nanoparticles Characterized with Light Scattering. Nanoscale Res. Lett. 2017, 12, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, N.Y.; Lee, J.; Kim, S.J.; Hong, S.H.; Park, H.M.; Yun, W.S.; Yoon, M.; Song, N.W. Preparation of an aqueous suspension of stabilized TiO2 nanoparticles in primary particle form. J. Nanosci. Nanotechnol. 2013, 13, 6153–6159. [Google Scholar] [CrossRef] [PubMed]
- Bielan, Z.; Dudziak, S.; Sulowska, A.; Pelczarski, D.; Ryl, J.; Zielińska-Jurek, A. Preparation and Characterization of Defective TiO2. The Effect of the Reaction Environment on Titanium Vacancies Formation. Materials 2020, 13, 2763. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S. An investigation on the long-term stability of TiO2 nanofluid. Mater. Today Proc. 2019, 11, 714–718. [Google Scholar] [CrossRef]
- Suttiponparnit, K.; Jiang, J.; Sahu, M.; Suvachittanont, S.; Charinpanitkul, T.; Biswas, P. Role of Surface Area, Primary Particle Size, and Crystal Phase on Titanium Dioxide Nanoparticle Dispersion Properties. Nanoscale Res Lett. 2011, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yin, L.; Tang, M.; Pu, Y. Optimized method for preparation of TiO2 nanoparticles dispersion for biological study. J. Nanosci. Nanotechnol. 2010, 10, 5213–5219. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, E.; Furusho, H.; Terasaki, O.; Palmqvist, A.E.C. Synthesis of nanoparticulate anatase and rutile crystallites at low temperatures in the Pluronic F127 microemulsion system. J. Mater. Res. 2011, 26, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Q.; Bastakoti, B.P.; Imura, M.; Hwang, S.M.; Sun, Z.Q.; Kim, J.H.; Dou, S.X.; Yamauchi, Y. Synthesis of mesoporous TiO2/SiO2 hybrid films as an efficient photocatalyst by polymeric micelle assembly. Chem. Eur. J. 2014, 20, 6027–6032. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wang, C.; Yan, Y. Synthesis of mesostructured titania with controlled crystalline framework. Chem. Mater. 2003, 15, 3841–3846. [Google Scholar] [CrossRef]
- Peng, T.; Zhao, D.; Dai, K.; Shi, W.; Hirao, K. Synthesis of titanium dioxide nanoparticles with mesoporous anatase wall and high photocatalytic activity. J. Phys. Chem. B 2005, 109, 4947–4952. [Google Scholar] [CrossRef] [PubMed]
- Smarsly, B.; Grosso, D.; Brezesinski, T.; Pinna, N.; Boissiere, C.; Antonietti, M.; Sanchez, C. Highly crystalline cubic mesoporous TiO2 with 10-nm pore diameter made with a new block copolymer template. Chem. Mater. 2008, 16, 2948–2952. [Google Scholar] [CrossRef]
- Kim, D.S.; Han, S.J.; Kwak, S.-Y. Synthesis and photocatalytic activity of mesoporous TiO2 with the surface area, crystallite size, and pore size. J. Colloid Interface Sci. 2007, 316, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, S.; Ho, G.W. Synthesis and tuning of ordering and crystallinity of mesoporous titanium dioxide film. Mater. Lett. 2009, 63, 1624–1627. [Google Scholar] [CrossRef]
- Gajjela, S.R.; Ananthanarayanan, K.; Yap, C.; Gratzel, M.; Balaya, P. Synthesis of mesoporous titanium dioxide by soft template based approach: Characterization and application in dye-sensitized solar cells. Energy Environ. Sci. 2010, 3, 838–845. [Google Scholar] [CrossRef]
- Deng, Y.; Cai, Y.; Sun, Z.; Liu, J.; Liu, C.; Wei, J.; Li, W.; Liu, C.; Wang, Y.; Zhao, D. Multifuntional mesoporous composite microsphere with well-designed nanostructure: A highly integrated catalyst system. J. Am. Chem. Soc. 2010, 132, 8466–8473. [Google Scholar] [CrossRef]
- Samsudin, E.M.; Abd Hamid, S.B.; Juan, J.C.; Basirun, W.J. Influence of triblock copolymer (pluronic F127) on enhancing the physico-chemical properties and photocatalytic response of mesoporous TiO2. Appl. Surf. Sci. 2015, 355, 959–968. [Google Scholar] [CrossRef]
- Suwanchawalit, C.; Wongnawa, S. Triblock copolymer-templated synthesis of porous TiO2 and its photocatalytic activity. J. Nanopart. Res. 2010, 12, 2895–2906. [Google Scholar] [CrossRef]
- Roy, S.; Ghosh, S.P.; Pradhan, D.; Sahu, P.K.; Kar, J.P. Morphological and electrical study of porous TiO2 films with various concentrations of Pluronic F-127 additive. J. Porous Mater. 2021, 28, 231–238. [Google Scholar] [CrossRef]
- Brown, W.; Schillén, K.; Almgren, M.; Hvidt, S.; Bahadur, P. Micelle and gel formation in a poly(ethylene oxide)/poly(propylene oxide)/poly(ethylene oxide) triblock copolymer in water solution: Dynamic and static light scattering and oscillatory shear measurements. J. Phys. Chem. 1991, 95, 1850–1858. [Google Scholar] [CrossRef]
- Wanka, G.; Hoffmann, H.; Ulbricht, W. Phase diagrams and aggregation behavior of poly(oxyethy1ene)-poly(oxypropylene)-poly(oxyethylene) triblock copolymers in aqueous solutions. Macromolecules 1994, 27, 4145–4159. [Google Scholar] [CrossRef]
- Alexandridis, P.; Hatton, T.A. Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Prudhomme, R.K.; Wu, G.; Schneider, D.K. Structure and rheology studies of poly(oxyethylene-oxypropylene-oxyethylene) aqueous solution. Langmuir 1996, 12, 4651–4659. [Google Scholar] [CrossRef]
- Malmsten, M.; Lindman, B. Self-Assembly in Aqueous Block Copolymer Solutions. Macromolecules 1992, 25, 5440–5445. [Google Scholar] [CrossRef]
- Mortensen, K. Structural studies of aqueous solutions of PEO–PPO–PEO triblock copolymers, their micellar aggregates and mesophases; a small-angle neutron scattering study. J. Phys. Condens. Matter 1996, 8, A103–A124. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of Poly(ethylene oxide)-Poly(propylene oxide)-Poly(ethylene oxide) Triblock Copolymers in Aqueous Solutions: Thermodynamics of Copolymer Association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Shaikhullina, M.; Khaliullina, A.; Gimatdinov, R.; Butakov, A.; Chernov, V.; Filippov, A. NMR relaxation and self-diffusion in aqueous micellar gels of pluronic F-127. J. Mol. Liq. 2020, 306, 112898. [Google Scholar] [CrossRef]
- Chaibundit, C.; Ricardo, N.M.P.S.; Costa, F.M.L.L.; Yeates, S.G.; Booth, C. Micellization and gelation of mixed copolymers P123 and F127 in aqueous solution. Langmuir 2007, 23, 9229–9236. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kabanov, A.V. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 2008, 130, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulthe, S.S.; Inamdar, N.N.; Choudhari, Y.M.; Shirolikar, S.M.; Borde, L.C.; Mourya, V.K. Mixed micelle formation with hydrophobic and hydrophilic Pluronic block copolymers: Implications for controlled and targeted drug delivery. Colloids Surf. B 2011, 88, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.I.; Lee, J.H.; Kim, J.-Y.; Kim, J.-C.; Kim, Y.H.; Tae, G. Efficient skin permeation of soluble proteins via flexible and functional nano-carrier. J. Control. Release 2012, 157, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Brunet-Maheu, J.M.; Fernandes, J.C.; De Lacerda, C.A.; Shi, Q.; Benderdour, M.; Lavigne, P. Pluronic F-127 as a Cell Carrier for Bone Tissue Engineering. J. Biomater. Appl. 2009, 24, 275–287. [Google Scholar] [CrossRef]
- Patel, H.S.; Shaikh, S.J.; Ray, D.; Aswal, V.K.; Vaidya, F.; Pathak, C.; Sharma, R.K. Formulation, solubilization, and in vitro characterization of quercetin-incorporated mixed micelles of PEO-PPO-PEO block copolymers. Appl. Biochem. Biotechnol. 2022, 194, 445–463. [Google Scholar] [CrossRef]
- Rahdar, A.; Hasanein, P.; Bilal, M.; Beyzaei, H.; Kyzas, G.Z. Quercetin-loaded F127 nanomicelles: Antioxidant activity and protection against renal injury induced by gentamicin in rats. Life Sci. 2021, 276, 119420. [Google Scholar] [CrossRef]
- Kassa, S.B.; Taslimi, P.; Özel, S.; Gür, B.; Gülçin, I.; Onganer, Y. Effects of some phenolic compounds on the inhibition of α-glycosidase enzyme-immobilized on Pluronic®F127 micelles: An in vitro and in silico study. Colloid Surf. A 2022, 632, 127839. [Google Scholar] [CrossRef]
- Rahdar, A.; Hajinezhad, M.R.; Barani, M.; Sargazi, S.; Zaboli, M.; Ghazy, E.; Baino, F.; Cucchiarini, M.; Bilal, M.; Pandey, S. Pluronic F127/Doxorubicin microemulsions: Preparation, characterization, and toxicity evaluations. J. Mol. Liq. 2022, 345, 117028. [Google Scholar] [CrossRef]
- Jiang, J.; Li, C.; Lombardi, J.; Colby, R.H.; Rigas, B.; Rafailovich, M.H.; Sokolov, J.C. The effect of physiologically relevant additives on the rheological properties of concentrated Pluronic copolymer gels. Polymer 2008, 49, 3561–3567. [Google Scholar] [CrossRef]
- Pradines, B.; Djabourov, M.; Vauthier, C.; Loiseau, P.M.; Ponchel, G.; Bouchemal, K. Gelation and micellization behaviors of pluronic®F127 hydrogel containing poly(isobutylcyanoacrylate) nanoparticles specifically designed for mucosal application. Colloids Surf. B 2015, 135, 669–676. [Google Scholar] [CrossRef]
- Dey, J.; Kumar, S.; Nath, S.; Ganguly, R.; Aswal, V.K.; Ismail, K. Additive induced core and corona specific dehydration and ensuing growth and interaction of Pluronic F127 micelles. J. Colloid Interface Sci. 2014, 415, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.; Cosgrove, T. Small-Angle Neutron Scattering Study of Adsorbed Pluronic Tri-Block Copolymers on Laponite. Langmuir 2005, 21, 9176–9182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gilstrap, K.; Wu, L.; Bahadur, R.; Moss, M.A.; Wang, Q.; Lu, X.; He, X. Synthesis and characterization of thermally responsive pluronic F127-chitosan nanocapsules for controlled release and intracellular delivery of small molecules. ACS Nano 2010, 4, 6747–6759. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.; Hebraud, P.; Gernigon, V.; Brochon, C.; Lapp, A.; Lindner, P.; Schlatter, G. Pluronic and β-cyclodextrin in water: From swollen micelles to self-assembled crystalline platelets. Soft Matter 2011, 7, 3502–3512. [Google Scholar] [CrossRef]
- Nambam, J.S.; Philip, J. Effects of Interaction of Ionic and Nonionic Surfactants on Self-Assembly of PEO−PPO−PEO Triblock Copolymer in Aqueous Solution. J. Phys. Chem. B 2012, 116, 1499–1507. [Google Scholar] [CrossRef]
- Branca, C.; Khouzami, K.; Wanderlingh, U.; D’Angelo, G. Effect of intercalated chitosan/clay nanostructures on concentrated pluronic F127 solution: A FTIR-ATR, DSC and rheological study. J. Colloid Interface Sci. 2018, 517, 221–229. [Google Scholar] [CrossRef]
- Branca, C.; D’Angelo, G. Aggregation behavior of Pluronic F127 solutions in presence of chitosan/clay nanocomposites examined by dynamic light scattering. J. Colloid Interface Sci. 2019, 542, 289–295. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Landau, L.D. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Progr. Surf. Sci. 1941, 14, 733–762. [Google Scholar] [CrossRef]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle processing: Understanding and controlling aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef]
- Branca, C.; Wanderlingh, U.; D’Angelo, G.; Crupi, C.; Rifici, S. Study of the dynamical behavior of sodium alginate/myoglobin aqueous solutions: A dynamic light scattering study. J. Mol. Liq. 2015, 209, 294–300. [Google Scholar] [CrossRef]
- Hassan, P.A.; Rana, S.; Verma, G. Making sense of brownian motion: Colloid characterization by dynamic light scattering. Langmuir 2015, 31, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics; John Wiley & Sons: New York, NY, USA, 1975. [Google Scholar]
- Finnie, K.M.; Cassidy, D.J.; Bartlett, J.R.; Woolfrey, J.L. IR Spectroscopy of Surface Water and Hydroxyl Species on Nanocrystalline TiO2 Films. Langmuir 2001, 17, 816–820. [Google Scholar] [CrossRef]
- Bedurftig, K.; Volkening, S.; Wang, Y.; Wintterlin, J.; Jacobi, K.; Ertl, G. Vibrational and structural properties of OH adsorbed on Pt(111). J. Chem. Phys. 1999, 111, 11147. [Google Scholar] [CrossRef]
- Su, Y.L.; Wang, J.; Liu, H.Z. FTIR spectroscopic investigation of effects of temperature and concentration on PEO-PPO-PEO block copolymer properties in aqueous solutions. Macromolecules 2002, 35, 6426–6431. [Google Scholar] [CrossRef]
- Su, Y.L.; Wang, J.; Liu, H.Z. Melt, hydration, and micellization of the PEO–PPO–PEO block copolymer studied by FTIR spectroscopy. J. Colloids Interface Sci. 2002, 251, 417–423. [Google Scholar] [CrossRef]
- Islam, M.R.; Shabani, B.; Rosengarten, G. Electrical and Thermal Conductivities of 50/50 Water-ethylene Glycol Based TiO2 Nanofluids to be Used as Coolants in PEM Fuel Cells. Energy Procedia 2017, 110, 101–108. [Google Scholar] [CrossRef]
- Chereches, E.I.; Minea, A.A. Electrical Conductivity of New Nanoparticle Enhanced Fluids: An Experimental Study. Nanomaterials 2019, 9, 1228. [Google Scholar] [CrossRef] [Green Version]
- Sikdar, S.; Basu, S.; Ganguly, S. Investigation of electrical conductivity of titanium dioxide nanofluids. Int. J. Nanopart. 2011, 4, 336–349. [Google Scholar] [CrossRef]







| Sample | Pluronic wt% | TiO2 wt% | pH | TiO2 (Mean Value) mg/mL |
|---|---|---|---|---|
| PAT-pH4 | 14 | 0.1 | 4 | 0.83 (0.083 wt%) |
| PAT-pH10 | 14 | 0.1 | 10 | 0.69 (0.069 wt%) |
| PBT-pH4 | 20 | 0.1 | 4 | 0.60 (0.060 wt%) |
| PBT-pH10 | 20 | 0.1 | 10 | 0.40 (0.040 wt%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti Nibali, V.; D’Angelo, G.; Arena, A.; Ciofi, C.; Scandurra, G.; Branca, C. TiO2 Nanoparticles Dispersion in Block-Copolymer Aqueous Solutions: Nanoarchitectonics for Self-Assembly and Aggregation. J. Funct. Biomater. 2022, 13, 39. https://doi.org/10.3390/jfb13020039
Conti Nibali V, D’Angelo G, Arena A, Ciofi C, Scandurra G, Branca C. TiO2 Nanoparticles Dispersion in Block-Copolymer Aqueous Solutions: Nanoarchitectonics for Self-Assembly and Aggregation. Journal of Functional Biomaterials. 2022; 13(2):39. https://doi.org/10.3390/jfb13020039
Chicago/Turabian StyleConti Nibali, Valeria, Giovanna D’Angelo, Antonella Arena, Carmine Ciofi, Graziella Scandurra, and Caterina Branca. 2022. "TiO2 Nanoparticles Dispersion in Block-Copolymer Aqueous Solutions: Nanoarchitectonics for Self-Assembly and Aggregation" Journal of Functional Biomaterials 13, no. 2: 39. https://doi.org/10.3390/jfb13020039
APA StyleConti Nibali, V., D’Angelo, G., Arena, A., Ciofi, C., Scandurra, G., & Branca, C. (2022). TiO2 Nanoparticles Dispersion in Block-Copolymer Aqueous Solutions: Nanoarchitectonics for Self-Assembly and Aggregation. Journal of Functional Biomaterials, 13(2), 39. https://doi.org/10.3390/jfb13020039