High-Precision 3D Printing of Microporous Cochlear Implants for Personalized Local Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Implant Design
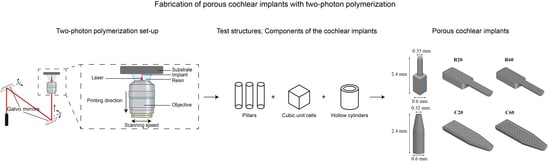
2.2. Preliminary Study: 3D Printing of Test Structures
2.3. 3D Printing of Cochlear Implants
2.4. 3D Printing of Samples for IP-Q Characterization
2.5. Morphological Characterization
2.6. Surface Topography
2.7. Chemical Characterization
2.8. Wettability and Surface Free Energy
2.9. Cytotoxicity
2.10. Image Analysis
2.11. Statistical Analysis
3. Results
3.1. 3D Printing of Pillar Arrays
3.2. 3D Printing of Cubic Unit Cells and Hollow Cylindrical Structures
3.3. 3D Printing of the Cochlear Implants
3.4. Morphological Characterization
3.5. Surface Topography
3.6. Chemical Characterization
3.7. Wettability and Surface Free Energy
3.8. Cytotoxicity
4. Discussion
4.1. Implant Design and Fabrication
4.2. Characterization
4.3. Outlook and Challenges
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, Q.; Guo, Q.; Zhu, M.; Zhang, J.; Chen, B.; Wu, T.; Jiang, W.; Tang, W. Application of Nanomedicine in Inner Ear Diseases. Front. Bioeng. Biotechnol. 2021, 9, 809443. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Hearing; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Laureyns, M.; Bisgaard, N.; Bobeldijk, M.; Zimmer, D. Getting the Numbers Right on Hearing Loss, Hearing Care and Hearing Aid Use in Europe Wide Strategy Joint AEA, EFHOH, EHIMA Report; EIMA: Brussels, Belgium, 2020. [Google Scholar]
- Ross, F.; Wohllebe, A. Evaluating the Service Quality of Mobile Health Versus Clinic Based Intervention in Hearing Healthcare. A Comparative Study. Int. J. Interact. Mob. Technol. 2021, 15, 21–32. [Google Scholar] [CrossRef]
- Naples, J.G.; Ruckenstein, M.J. Cochlear Implant. Otolaryngol. Clin. N. Am. 2020, 53, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Chakravorti, S.; Noble, J.H.; Gifford, R.H.; Dawant, B.M.; O’Connell, B.P.; Wang, J.; Labadie, R.F. Further Evidence of the Relationship Between Cochlear Implant Electrode Positioning and Hearing Outcomes. Otol. Neurotol. 2019, 40, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Altissimi, G.; Colizza, A.; Cianfrone, G.; de Vincentiis, M.; Greco, A.; Taurone, S.; Musacchio, A.; Ciofalo, A.; Turchetta, R.; Angeletti, D.; et al. Drugs Inducing Hearing Loss, Tinnitus, Dizziness and Vertigo: An Updated Guide. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7946–7952. [Google Scholar] [CrossRef] [PubMed]
- Piu, F.; Bishop, K.M. Local Drug Delivery for the Treatment of Neurotology Disorders. Front. Cell. Neurosci. 2019, 13, 238. [Google Scholar] [CrossRef]
- McCall, A.A.; Swan, E.E.; Borenstein, J.T.; Sewell, W.F.; Kujawa, S.G.; McKenna, M.J. Drug Delivery for Treatment of Inner Ear Disease: Current State of Knowledge. Ear Hear. 2010, 31, 156–165. [Google Scholar] [CrossRef]
- Carvalho; Gerard, J.; Anil, K. Lalwani The Effect of Cochleostomy and Intracochlear Infusion on Auditory Brain Stem Response Threshold in the Guinea Pig. Am. J. Otol. 1999, 20, 87–90. [Google Scholar]
- Krenzlin, S.; Vincent, C.; Munzke, L.; Gnansia, D.; Siepmann, J.; Siepmann, F. Predictability of drug release from cochlear implants. J. Control. Release 2012, 159, 60–68. [Google Scholar] [CrossRef]
- Gehrke, M.; Sircoglou, J.; Gnansia, D.; Tourrel, G.; Willart, J.F.; Danede, F.; Lacante, E.; Vincent, C.; Siepmann, F.; Siepmann, J. Ear Cubes for local controlled drug delivery to the inner ear. Int. J. Pharm. 2016, 509, 85–94. [Google Scholar] [CrossRef]
- Lehner, E.; Gündel, D.; Liebau, A.; Plontke, S.; Mäder, K. Intracochlear PLGA Based Implants for Dexamethasone Release: Challenges and Solutions. Int. J. Pharm. X 2019, 1, 100015. [Google Scholar] [CrossRef] [PubMed]
- Wachowiak, S.; Danede, F.; Willart, J.F.; Siepmann, F.; Siepmann, J.; Hamoudi, M. PLGA Implants for Controlled Dexamethasone Delivery: Impact of the Polymer Chemistry. J. Drug Deliv. Sci. Technol. 2023, 86, 104648. [Google Scholar] [CrossRef]
- Rongthong, T.; Qnouch, A.; Gehrke, M.M.; Danede, F.; Willart, J.F.; de Oliveira, P.F.M.; Paccou, L.; Tourrel, G.; Stahl, P.; Verin, J.; et al. Long Term Behavior of Dexamethasone-Loaded Cochlear Implants: In Vitro & in Vivo. Int. J. Pharm. X 2022, 4, 100141. [Google Scholar] [CrossRef] [PubMed]
- Rongthong, T.; Qnouch, A.; Maue Gehrke, M.; Paccou, L.; Oliveira, P.; Danede, F.; Verin, J.; Vincent, C.; Willart, J.-F.; Siepmann, F.; et al. Silicone Matrices for Controlled Dexamethasone Release: Toward a Better Understanding of the Underlying Mass Transport Mechanisms. Regen. Biomater. 2023, 10, rbad008. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Viaño, I.; Trenfield, S.J.; Basit, A.W.; Goyanes, A. Translating 3D Printed Pharmaceuticals: From Hype to Real-World Clinical Applications. Adv. Drug Deliv. Rev. 2021, 174, 553–575. [Google Scholar] [CrossRef]
- Wu, C.; Luo, Y.; Cuniberti, G.; Xiao, Y.; Gelinsky, M. Three-Dimensional Printing of Hierarchical and Tough Mesoporous Bioactive Glass Scaffolds with a Controllable Pore Architecture, Excellent Mechanical Strength and Mineralization Ability. Acta Biomater. 2011, 7, 2644–2650. [Google Scholar] [CrossRef]
- Zhu, M.; Li, K.; Zhu, Y.; Zhang, J.; Ye, X. 3D-Printed Hierarchical Scaffold for Localized Isoniazid/Rifampin Drug Delivery and Osteoarticular Tuberculosis Therapy. Acta Biomater. 2015, 16, 145–155. [Google Scholar] [CrossRef]
- Narayan, R.J.; Doraiswamy, A.; Chrisey, D.B.; Chichkov, B.N. Medical Prototyping Using Two Photon Polymerization. Mater. Today 2010, 13, 42–48. [Google Scholar] [CrossRef]
- Bassand, C.; Benabed, L.; Charlon, S.; Verin, J.; Freitag, J.; Siepmann, F.; Soulestin, J.; Siepmann, J. 3D Printed PLGA Implants: APF DDM vs. FDM. J. Control. Release 2023, 353, 864–874. [Google Scholar] [CrossRef]
- Goyanes, A.; Madla, C.M.; Umerji, A.; Duran Piñeiro, G.; Giraldez Montero, J.M.; Lamas Diaz, M.J.; Gonzalez Barcia, M.; Taherali, F.; Sánchez-Pintos, P.; Couce, M.-L.; et al. Automated Therapy Preparation of Isoleucine Formulations Using 3D Printing for the Treatment of MSUD: First Single-Centre, Prospective, Crossover Study in Patients. Int. J. Pharm. 2019, 567, 118497. [Google Scholar] [CrossRef]
- Januskaite, P.; Xu, X.; Ranmal, S.R.; Gaisford, S.; Basit, A.W.; Tuleu, C.; Goyanes, A. I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3D Printed Tablets. Pharmaceutics 2020, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Scarpa, M.; Kamlow, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Patient Acceptability of 3D Printed Medicines. Int. J. Pharm. 2017, 530, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective Laser Sintering (SLS) 3D Printing of Medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.J.N.; Yong, W.P.; Kochhar, J.S.; Khanolkar, J.; Yao, X.; Sun, Y.; Ao, C.K.; Soh, S. On-Demand Fully Customizable Drug Tablets via 3D Printing Technology for Personalized Medicine. J. Control. Release 2020, 322, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Müllertz, A.; Rantanen, J. Additive Manufacturing of Solid Products for Oral Drug Delivery Using Binder Jetting Three-Dimensional Printing. AAPS PharmSciTech 2022, 23, 196. [Google Scholar] [CrossRef]
- Dong, M.; Wang, X.; Chen, X.-Z.; Mushtaq, F.; Deng, S.; Zhu, C.; Torlakcik, H.; Terzopoulou, A.; Qin, X.-H.; Xiao, X.; et al. 3D-Printed Soft Magnetoelectric Microswimmers for Delivery and Differentiation of Neuron-Like Cells. Adv. Funct. Mater. 2020, 30, 1910323. [Google Scholar] [CrossRef]
- Bozuyuk, U.; Yasa, O.; Yasa, I.C.; Ceylan, H.; Kizilel, S.; Sitti, M. Light-Triggered Drug Release from 3D-Printed Magnetic Chitosan Microswimmers. ACS Nano 2018, 12, 9617–9625. [Google Scholar] [CrossRef]
- Faraji Rad, Z.; Prewett, P.D.; Davies, G.J. High-Resolution Two-Photon Polymerization: The Most Versatile Technique for the Fabrication of Microneedle Arrays. Microsyst. Nanoeng. 2021, 7, 71. [Google Scholar] [CrossRef]
- He, G.S.; Tan, L.-S.; Zheng, Q.; Prasad, P.N. Multiphoton Absorbing Materials: Molecular Designs, Characterizations, and Applications. Chem. Rev. 2008, 108, 1245–1330. [Google Scholar] [CrossRef]
- He, G.S.; Xu, G.C.; Prasad, P.N.; Reinhardt, B.A.; Bhatt, J.C.; Dillard, A.G. Two-Photon Absorption and Optical-Limiting Properties of Novel Organic Compounds. Opt. Lett. 1995, 20, 435–437. [Google Scholar] [CrossRef]
- Tan, D.; Li, Y.; Qi, F.; Yang, H.; Gong, Q.; Dong, X.; Duan, X. Reduction in Feature Size of Two-Photon Polymerization Using SCR500. Appl. Phys. Lett. 2007, 90, 71106. [Google Scholar] [CrossRef]
- Geng, Q.; Wang, D.; Chen, P.; Chen, S.-C. Ultrafast Multi-Focus 3-D Nano-Fabrication Based on Two-Photon Polymerization. Nat. Commun. 2019, 10, 2179. [Google Scholar] [CrossRef]
- Stichel, T.; Hecht, B.; Houbertz, R.; Sextl, G. Two-Photon Polymerization as Method for the Fabrication of Large Scale Biomedical Scaffold Applications. J. Laser Micro/Nanoeng. 2010, 5, 209–212. [Google Scholar] [CrossRef]
- Mckee, S.; Lutey, A.; Sciancalepore, C.; Poli, F.; Selleri, S.; Cucinotta, A. Microfabrication of Polymer Microneedle Arrays Using Two-Photon Polymerization. J. Photochem. Photobiol. B 2022, 229, 112424. [Google Scholar] [CrossRef]
- Weiss, T.; Hildebrand, G.; Schade, R.; Liefeith, K. Two-Photon Polymerization for Microfabrication of Three-Dimensional Scaffolds for Tissue Engineering Application. Eng. Life Sci. 2009, 9, 384–390. [Google Scholar] [CrossRef]
- Sharaf, A.; Roos, B.; Timmerman, R.; Kremers, G.-J.; Bajramovic, J.J.; Accardo, A. Two-Photon Polymerization of 2.5D and 3D Microstructures Fostering a Ramified Resting Phenotype in Primary Microglia. Front. Bioeng. Biotechnol. 2022, 10, 926642. [Google Scholar] [CrossRef]
- Rananaware, P.; Brahmkhatria, V.P.; Dasgupta, D.; Patel, A. Functionalized Mesoporous Silica for Drug Delivery of Poorly Soluble Polyphenols: Synthesis, Characterization, and Antimicrobial Action. J. Solid State Chem. 2023, 326, 124214. [Google Scholar] [CrossRef]
- Yang, Y.; Qiao, X.; Huang, R.; Chen, H.; Shi, X.; Wang, J.; Tan, W.; Tan, Z. E-Jet 3D Printed Drug Delivery Implants to Inhibit Growth and Metastasis of Orthotopic Breast Cancer. Biomaterials 2020, 230, 119618. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, M.; Gopinadhan, K.; Han, S.A.; Saha, S.; Park, H.J.; Cho, E.B.; Kumar, B.; Patra, A.; Kim, S.-W.; Venkatesan, T. Surface Energy and Wettability of van Der Waals Structures. Nanoscale 2016, 8, 5764–5770. [Google Scholar] [CrossRef]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Rabel, W. Einige Aspekte Der Benetzungstheorie Und Ihre Anwendung Auf Die Untersuchung Und Veränderung Der Oberflächeneigenschaften von Polymeren. Farbe Und Lack 1971, 77, 997–1005. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Abdel–Fattah, E. Surface Activation of Poly(Methyl Methacrylate) with Atmospheric Pressure Ar + H2O Plasma. Coatings 2019, 9, 228. [Google Scholar] [CrossRef]
- Hu, Q.; Rance, G.A.; Trindade, G.F.; Pervan, D.; Jiang, L.; Foerster, A.; Turyanska, L.; Tuck, C.; Irvine, D.J.; Hague, R.; et al. The Influence of Printing Parameters on Multi-Material Two-Photon Polymerisation Based Micro Additive Manufacturing. Addit. Manuf. 2022, 51, 102575. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Wan, X.; Li, N.; Li, J.; Yin, Q.; Zhang, L. High-Efficiency Two-Photon Polymerization Initiators Based on Coumarin Derivatives for Additive Manufacturing with Sub-Wavelength Resolution. Mater. Today Chem. 2023, 30, 101599. [Google Scholar] [CrossRef]
- Davidson, C.L.; Feilzer, A.J. Polymerization Shrinkage and Polymerization Shrinkage Stress in Polymer-Based Restoratives. J. Dent. 1997, 25, 435–440. [Google Scholar] [CrossRef]
- Kamranikia, K.; Dominici, S.; Keller, M.; Kube, N.; Mougin, K.; Spangenberg, A. Very High-Aspect-Ratio Polymeric Micropillars Made by Two-Photon Polymerization. Micromachines 2023, 14, 1602. [Google Scholar] [CrossRef]
- Zhou, X.; Hou, Y.; Lin, J. A Review on the Processing Accuracy of Two-Photon Polymerization. AIP Adv. 2015, 5, 030701. [Google Scholar] [CrossRef]
- Kim, H.; Pingali, R.; Saha, S.K. Rapid Printing of Nanoporous 3D Structures by Overcoming the Proximity Effects in Projection Two-Photon Lithography. Virtual Phys. Prototyp. 2023, 18, e2230979. [Google Scholar] [CrossRef]
- Nguyen, A.K.; Narayan, R.J. Two-Photon Polymerization for Biological Applications. Mater. Today 2017, 20, 314–322. [Google Scholar] [CrossRef]
- Nouri-Goushki, M.; Isaakidou, A.; Eijkel, B.I.M.; Minneboo, M.; Liu, Q.; Boukany, P.E.; Mirzaali, M.J.; Fratila-Apachitei, L.E.; Zadpoor, A.A. 3D Printed Submicron Patterns Orchestrate the Response of Macrophages. Nanoscale 2021, 13, 14304–14315. [Google Scholar] [CrossRef] [PubMed]
- Udenni Gunathilake, T.M.S.; Ching, Y.C.; Ching, K.Y.; Chuah, C.H.; Abdullah, L.C. Biomedical and Microbiological Applications of Bio-Based Porous Materials: A Review. Polymers 2017, 9, 160. [Google Scholar] [CrossRef]
- Li, Z.; Xu, P.; Shang, L.; Ma, B.; Zhang, H.; Fu, L.; Ou, Y.; Mao, Y. 3D Collagen Porous Scaffold Carrying PLGA-PTX/SDF-1α Recruits and Promotes Neural Stem Cell Differentiation for Spinal Cord Injury Repair. J. Biomater. Sci. Polym. Ed. 2023, 34, 1–24. [Google Scholar] [CrossRef]
- El-Wakil, N.; Kamel, R.; Mahmoud, A.A.; Dufresne, A.; Abouzeid, R.E.; Abo El-Fadl, M.T.; Maged, A. Risedronate-Loaded Aerogel Scaffolds for Bone Regeneration. Drug Deliv. 2023, 30, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Qiang, L.; Fan, M.; Liu, Y.; Yang, A.; Chang, D.; Li, J.; Sun, T.; Wang, Y.; Guo, R.; et al. 3D-Printed Tri-Element-Doped Hydroxyapatite/Polycaprolactone Composite Scaffolds with Antibacterial Potential for Osteosarcoma Therapy and Bone Regeneration. Bioact. Mater. 2024, 31, 18–37. [Google Scholar] [CrossRef]
- Sravani, K.G.; Desala, R.K.; Chand, P.; Sathvik, K.; Rao, K.S.; Lay-Ekuakille, A. Design and Analysis of Bio-Inspired Micro-Needle for Drug Delivery Applications. IEEE Trans. Nanobiosci. 2023, 22, 237–244. [Google Scholar] [CrossRef]
- Liu, L.; Kai, H.; Nagamine, K.; Ogawa, Y.; Nishizawa, M. Porous Polymer Microneedles with Interconnecting Microchannels for Rapid Fluid Transport. RSC Adv. 2016, 6, 48630–48635. [Google Scholar] [CrossRef]
- Dewaele, M.; Truffier-Boutry, D.; Devaux, J.; Leloup, G. Volume Contraction in Photocured Dental Resins: The Shrinkage-Conversion Relationship Revisited. Dent. Mater. 2006, 22, 359–365. [Google Scholar] [CrossRef]
- Bauer, J.; Guell Izard, A.; Zhang, Y.; Baldacchini, T.; Valdevit, L. Programmable Mechanical Properties of Two-Photon Polymerized Materials: From Nanowires to Bulk. Adv. Mater. Technol. 2019, 4, 1900146. [Google Scholar] [CrossRef]
- Jiang, L.J.; Zhou, Y.S.; Xiong, W.; Gao, Y.; Huang, X.; Jiang, L.; Baldacchini, T.; Silvain, J.-F.; Lu, Y.F. Two-Photon Polymerization: Investigation of Chemical and Mechanical Properties of Resins Using Raman Microspectroscopy. Opt. Lett. 2014, 39, 3034–3037. [Google Scholar] [CrossRef] [PubMed]
- Pianelli, C.; Devaux, J.; Bebelman, S.; Leloup, G. The Micro-Raman Spectroscopy, a Useful Tool to Determine the Degree of Conversion of Light-Activated Composite Resins. J. Biomed. Mater. Res. 1999, 48, 675–681. [Google Scholar] [CrossRef]
- Schweiger, S.; Schulze, T.; Schlipf, S.; Reinig, P.; Schenk, H. Characterization of Two-Photon-Polymerization Lithography Structures via Raman Spectroscopy and Nanoindentation. J. Opt. Microsyst. 2022, 2, 33501. [Google Scholar] [CrossRef]
- Zeiger, A.S.; Hinton, B.; Van Vliet, K.J. Why the Dish Makes a Difference: Quantitative Comparison of Polystyrene Culture Surfaces. Acta Biomater. 2013, 9, 7354–7361. [Google Scholar] [CrossRef]
- Havenith, S.; Versnel, H.; Agterberg, M.J.H.; de Groot, J.C.M.J.; Sedee, R.-J.; Grolman, W.; Klis, S.F.L. Spiral Ganglion Cell Survival after Round Window Membrane Application of Brain-Derived Neurotrophic Factor Using Gelfoam as Carrier. Hear. Res. 2011, 272, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, H.; Chang, A.; Kel, G.; Sly, D.; Richardson, R.; O’Leary, S.J. Round Window Delivery of Dexamethasone Ameliorates Local and Remote Hearing Loss Produced by Cochlear Implantation into the Second Turn of the Guinea Pig Cochlea. Hear. Res. 2010, 265, 25–29. [Google Scholar] [CrossRef]
- Saber, A.; Strand, S.P.; Ulfendahl, M. Use of the Biodegradable Polymer Chitosan as a Vehicle for Applying Drugs to the Inner Ear. Eur. J. Pharm. Sci. 2010, 39, 110–115. [Google Scholar] [CrossRef]
- Lee, M.Y.; Lee, J.H.; Lee, H.S.; Choi, J.-J.; Jang, J.; Choi, H.; Oh, S.-H.; Jang, J.H. Continuous Topical Drug Delivery Using Osmotic Pump in Animal Cochlear Implant Model: Continuous Steroid Delivery Is Effective for Hearing Preservation. Acta Otolaryngol. 2015, 135, 791–798. [Google Scholar] [CrossRef]
- Jang, J.; Kim, J.; Kim, Y.C.; Kim, S.; Chou, N.; Lee, S.; Choung, Y.; Kim, S.; Brugger, J.; Choi, H.; et al. A 3D Microscaffold Cochlear Electrode Array for Steroid Elution. Adv. Heal. Mater. 2019, 8, 1900379. [Google Scholar] [CrossRef]
- Toulemonde, P.; Risoud, M.; Lemesre, P.E.; Beck, C.; Wattelet, J.; Tardivel, M.; Siepmann, J.; Vincent, C. Evaluation of the Efficacy of Dexamethasone-Eluting Electrode Array on the Post-Implant Cochlear Fibrotic Reaction by Three-Dimensional Immunofluorescence Analysis in Mongolian Gerbil Cochlea. J. Clin. Med. 2021, 10, 3315. [Google Scholar] [CrossRef]
- Tamura, T.; Kita, T.; Nakagawa, T.; Endo, T.; Kim, T.-S.; Ishihara, T.; Mizushima, Y.; Higaki, M.; Ito, J. Drug Delivery to the Cochlea Using PLGA Nanoparticles. Laryngoscope 2005, 115, 2000–2005. [Google Scholar] [CrossRef]
- Dang, H.P.; Shabab, T.; Shafiee, A.; Peiffer, Q.C.; Fox, K.; Tran, N.; Dargaville, T.R.; Hutmacher, D.W.; Tran, P.A. 3D Printed Dual Macro-, Microscale Porous Network as a Tissue Engineering Scaffold with Drug Delivering Function. Biofabrication 2019, 11, 35014. [Google Scholar] [CrossRef] [PubMed]
- Gomes Filho, M.S.; Oliveira, F.A.; Barbosa, M.A.A. A Statistical Mechanical Model for Drug Release: Investigations on Size and Porosity Dependence. Phys. A Stat. Mech. Its Appl. 2016, 460, 29–37. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical Modeling of Drug Delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]





| Pillar Array | Cubic Unit Cell | Hollow Cylinder | |
|---|---|---|---|
 |  |  | |
| Geometrical characteristics | l = 80 μm d = 5 μm in = 5 μm + (n − 1), for 1 ≤ n ≤ 16 | l = 240 μm s = 20, 30, 40, 50 μm | l = 240 μm w = 20, 30, 40, 50, 75, 100 μm |
| 2PP printing parameters | LP = 25, 40, 50 mW υ = 150,000 μm/s s = 5 μm h = 1 μm | ||
| R20 | R60 | C20 | C60 | |
|---|---|---|---|---|
| Volume of the implant shell, VS (mm3) | 0.15 | 0.15 | 0.19 | 0.19 |
| Volume of the solid insert, VI (mm3) | 0.41 | 0.41 | 0.33 | 0.33 |
| Volume of the lattice, VL (mm3) | 0.22 | 0.20 | 0.16 | 0.16 |
| Porosity, φ (%) | 46 | 52 | 50 | 50 |
| Designed s (μm) | Designed w (μm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 30 | 40 | 50 | 20 | 30 | 40 | 50 | 75 | 100 | |
| LP (mW) | Resulting s (μm) | Resulting w (μm) | ||||||||
| 25 | × | × | × | 25.20 ± 0.84 | × | × | × | × | 40.64 ± 0.0 | × |
| 40 | × | × | 20.39 ± 1.33 | 29.65 ± 1.39 | × | 36.76 ± 7.34 | 52.59 ± 4.39 | 63.90 ± 3.46 | 97.06 ± 2.30 | × |
| 50 | × | 17.57 ± 1.17 | 26.48 ± 1.79 | 35.43 ± 1.25 | × | 43.67 ± 3.71 | 62.57 ± 4.02 | 72.70 ± 7.47 | 108.68 ± 6.13 | × |
| Parameter | Pore Type | |
|---|---|---|
| 20 | 60 | |
| p | 17.88 ± 0.95 | 58.15 ± 1.62 |
| w | 64.08 ± 1.76 | 72.50 ± 3.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isaakidou, A.; Apachitei, I.; Fratila-Apachitei, L.E.; Zadpoor, A.A. High-Precision 3D Printing of Microporous Cochlear Implants for Personalized Local Drug Delivery. J. Funct. Biomater. 2023, 14, 494. https://doi.org/10.3390/jfb14100494
Isaakidou A, Apachitei I, Fratila-Apachitei LE, Zadpoor AA. High-Precision 3D Printing of Microporous Cochlear Implants for Personalized Local Drug Delivery. Journal of Functional Biomaterials. 2023; 14(10):494. https://doi.org/10.3390/jfb14100494
Chicago/Turabian StyleIsaakidou, Aikaterini, Iulian Apachitei, Lidy Elena Fratila-Apachitei, and Amir Abbas Zadpoor. 2023. "High-Precision 3D Printing of Microporous Cochlear Implants for Personalized Local Drug Delivery" Journal of Functional Biomaterials 14, no. 10: 494. https://doi.org/10.3390/jfb14100494
APA StyleIsaakidou, A., Apachitei, I., Fratila-Apachitei, L. E., & Zadpoor, A. A. (2023). High-Precision 3D Printing of Microporous Cochlear Implants for Personalized Local Drug Delivery. Journal of Functional Biomaterials, 14(10), 494. https://doi.org/10.3390/jfb14100494