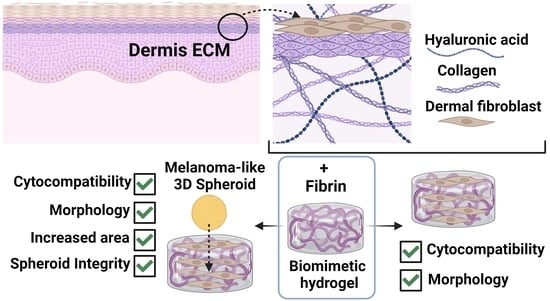
Bioinspired Collagen/Hyaluronic Acid/Fibrin-Based Hydrogels for Soft Tissue Engineering: Design, Synthesis, and In Vitro Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CHAF Hydrogels
2.3. Characterization of CHAF Hydrogels
2.3.1. Quantification of the Crosslinking Degree
2.3.2. Morphological Analysis
2.3.3. Rheological Analysis
2.3.4. Gelation Time
2.3.5. Fourier Transform Infrared-Attenuated TR
2.3.6. Nutrient Diffusion
2.3.7. Water Uptake and Water Content
2.3.8. Degradation
2.4. Preparation of Sterile CHAF Hydrogels
2.5. Cytocompatibility and Biological Characterisation Using Human Fibroblasts
2.5.1. 2D Seeding and Embedding of Fibroblasts into CHAF_1 Hydrogel
2.5.2. PrestoBlue Assay
2.5.3. Live and Dead Assay
2.5.4. Cell Morphology by Fluorescence Microscopy
2.6. Formation of Melanoma Spheroids and Culture within the CHAF_1 Hydrogel
2.6.1. CellTiter-Glo® 3D Cell Viability Assay
2.6.2. Morphological Analysis of 3D Cultures
2.7. Statistical Analysis
3. Results
3.1. Characterization of Collagen/Hyaluronic Acid/Fibrin (CHAF) Hydrogels
3.2. Collagen/Hyaluronic Acid/Fibrin (CHAF_1) Cytocompatibility Assessment
3.3. Melanoma Spheroids Morphological Analysis and Encapsulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atala, A. Advances in Tissue and Organ Replacement. Curr. Stem Cell Res. Ther. 2008, 3, 21–31. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Ruedinger, F.; Lavrentieva, A.; Blume, C.; Pepelanova, I.; Scheper, T. Hydrogels for 3D mammalian cell culture: A starting guide for laboratory practice. Appl. Microbiol. Biotechnol. 2014, 99, 623–636. [Google Scholar] [CrossRef]
- McLafferty, E.; Hendry, C.; Farley, A. The integumentary system: Anatomy, physiology and function of skin. Nurs. Stand. 2012, 27, 35. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Shin, H.; Lim, D.W. Biomimetic Scaffolds for Tissue Engineering. Adv. Funct. Mater. 2012, 22, 2446–2468. [Google Scholar] [CrossRef]
- MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef]
- Pfisterer, K.; Shaw, L.E.; Symmank, D.; Weninger, W. The extracellular matrix in skin inflammation and infection. Front. Cell Dev. Biol. 2011, 9, 682414. [Google Scholar] [CrossRef]
- Tan, S.H.; Chua, D.A.C.; Tang, J.R.J.; Bonnard, C.; Leavesley, D.; Liang, K. Design of hydrogel-based scaffolds for in vitro three-dimensional human skin model reconstruction. Acta Biomater. 2022, 153, 13–37. [Google Scholar] [CrossRef]
- Kwang-Hun, J.; Park, D.; Lee, Y.-C. Polymer-based hydrogel scaffolds for skin tissue engineering applications: A mini-review. J. Polym. Res. 2017, 24, 112. [Google Scholar]
- Gsib, O.; Egles, C.; Bencherif, S.A. Fibrin: An underrated biopolymer for skin tissue engineering. J. Mol. Biol. Biotech 2017, 2, 3. [Google Scholar]
- Dash, B.C.; Duan, K.; Xing, H.; Kyriakides, T.R.; Hsia, H.C. An in situ collagen-HA hydrogel system promotes survival and preserves the proangiogenic secretion of hiPSC-derived vascular smooth muscle cells. Biotechnol. Bioeng. 2020, 117, 3912–3923. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Thushara, R.M.; Chandranayaka, S.; Sherman, L.S.; Kemparaju, K.; Girish, K.S. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016, 86, 917–928. [Google Scholar] [CrossRef]
- Gaspar, A.; Moldovan, L.; Constantin, D.; Stanciuc, A.; Boeti, P.S.; Efrimescu, I. Collagen–based scaffolds for skin tissue engineering. J. Med. Life 2011, 4, 172–177. [Google Scholar] [PubMed]
- Park, C.H.; Woo, K.M. Fibrin-based biomaterial applications in tissue engineering and regenerative medicine. Biomim. Med. Mater. Nanotechnol. 3D Bioprinting 2018, 1064, 253–261. [Google Scholar]
- Montero, A.; Atienza, C.; Elvira, C.; Jorcano, J.L.; Velasco, D. Hyaluronic acid-fibrin hydrogels show improved mechanical stability in dermo-epidermal skin substitutes. Mater. Sci. Eng. C 2021, 128, 112352. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Barthold, C.; Baganz, S.; Wilhelmi, M.; Scheper, T.; Pepelanova, I. Hydrogels based on collagen and fibrin—frontiers and applications. BioNanoMaterials 2016, 17, 3–12. [Google Scholar] [CrossRef]
- Ying, H.; Zhou, J.; Wang, M.; Su, D.; Ma, Q.; Lv, G.; Chen, J. In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressing for promoting spontaneous wound healing. Mater. Sci. Eng. C 2019, 101, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Dare, E.V.; Hincke, M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-W.; Zhang, X.-W.; Mi, C.-H.; Qi, X.-Y.; Zhou, J.; Wei, D.-X. Recent advances in hyaluronic acid-based hydrogels for 3D bioprinting in tissue engineering applications. Smart Mater. Med. 2022, 4, 59–68. [Google Scholar] [CrossRef]
- Liao, J.; Jia, Y.; Wang, B.; Shi, K.; Qian, Z. Injectable hybrid poly (ε-caprolactone)-b-poly (ethylene glycol)-b-poly (ε-caprolactone) porous microspheres/alginate hydrogel cross-linked by calcium gluconate crystals deposited in the pores of microspheres improved skin wound healing. ACS Biomater. Sci. Eng. 2018, 4, 1029–1036. [Google Scholar] [CrossRef]
- Mehra, T.D.; Ghosh, K.; Shu, X.Z.; Prestwich, G.D.; Clark, R.A. Molecular Stenting with a Crosslinked Hyaluronan Derivative Inhibits Collagen Gel Contraction. J. Investig. Dermatol. 2006, 126, 2202–2209. [Google Scholar] [CrossRef] [PubMed]
- Godesky, M.D.; Shreiber, D.I. Hyaluronic acid-based hydrogels with independently tunable mechanical and bioactive signaling features. Biointerphases 2019, 14, 061005. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fukunaga-Kalabis, M.; Herlyn, M. The three-dimensional human skin reconstruct model: A tool to study normal skin and melanoma progression. J. Vis. Exp. 2011, 54, e2937. [Google Scholar]
- Eves, P.; Layton, C.; Hedley, S.; Dawson, R.; Wagner, M.; Morandini, R.; Ghanem, G.; Mac Neil, S. Characterization of an in vitro model of human melanoma invasion based on reconstructed human skin. Br. J. Dermatol. 2000, 142, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Lotz, C.; Schmid, F.F.; Oechsle, E.; Monaghan, M.G.; Walles, H.; Groeber-Becker, F. Cross-linked collagen hydrogel matrix resisting contraction to facilitate full-thickness skin equivalents. ACS Appl. Mater. Interfaces 2017, 9, 20417–20425. [Google Scholar] [CrossRef]
- Ramasamy, S.; Davoodi, P.; Vijayavenkataraman, S.; Teoh, J.H.; Thamizhchelvan, A.M.; Robinson, K.S.; Wu, B.; Fuh, J.Y.; DiColandrea, T.; Zhao, H.; et al. Optimized construction of a full thickness human skin equivalent using 3D bioprinting and a PCL/collagen dermal scaffold. Bioprinting 2021, 21, e00123. [Google Scholar] [CrossRef]
- Müller, I.; Kulms, D. A 3D Organotypic Melanoma Spheroid Skin Model. J. Vis. Exp. 2018, 135, e57500. [Google Scholar] [CrossRef]
- da Conceicao Ribeiro, R.; Pal, D.; Ferreira, A.M.; Gentile, P.; Benning, M.; Dalgarno, K. Reactive jet impingement bioprinting of high cell density gels for bone microtissue fabrication. Biofabrication 2018, 11, 015014. [Google Scholar] [CrossRef]
- Sadat, A.; Joye, I.J. Peak Fitting Applied to Fourier Transform Infrared and Raman Spectroscopic Analysis of Proteins. Appl. Sci. 2020, 10, 5918. [Google Scholar] [CrossRef]
- De Campos Vidal, B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef]
- Belbachir, K.; Noreen, R.; Gouspillou, G.; Petibois, C. Collagen types analysis and differentiation by FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, G.; Toumpaniari, S.; Popov, A.; Duan, P.; Chen, J.; Dalgarno, K.; Scott, W.; Ferreira, A. Synthesis of bioinspired collagen/alginate/fibrin based hydrogels for soft tissue engineering. Mater. Sci. Eng. C 2018, 91, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Mirzayeva, T.; Čopíková, J.; Kvasnička, F.; Bleha, R.; Synytsya, A. Screening of the Chemical Composition and Identification of Hyaluronic Acid in Food Supplements by Fractionation and Fourier-Transform Infrared Spectroscopy. Polymers 2021, 13, 4002. [Google Scholar] [CrossRef]
- Pan, N.C.; Pereira, H.C.B.; Silva, M.d.L.C.d.; Vasconcelos, A.F.D.; Celligoi, M.A.P.C. Improvement Production of Hyaluronic Acid by Streptococcus zooepidemicus in Sugarcane Molasses. Appl. Biochem. Biotechnol. 2016, 182, 276–293. [Google Scholar] [CrossRef]
- Chen, H.; Qin, J.; Hu, Y. Efficient degradation of highmolecular-weight hyaluronic acid by a combination of ultrasound, hydrogen peroxide, and copper ion. Molecules 2019, 24, 617. [Google Scholar] [CrossRef]
- Sizeland, K.H.; Hofman, K.A.; Hallett, I.C.; Martin, D.E.; Potgieter, J.; Kirby, N.M.; Hawley, A.; Mudie, S.T.; Ryan, T.M.; Haverkamp, R.G.; et al. Nanostructure of electrospun collagen: Do electrospun collagen fibers form native structures? Materialia 2018, 3, 90–96. [Google Scholar] [CrossRef]
- Deepthi, S.; Jayakumar, R. Alginate nanobeads interspersed fibrin network as in situ forming hydrogel for soft tissue engineering. Bioact. Mater. 2018, 3, 194–200. [Google Scholar] [CrossRef]
- Litvinov, R.I.; Faizullin, D.A.; Zuev, Y.F.; Weisel, J.W. The α-helix to β-sheet transition in stretched and compressed hydrated fibrin clots. Biophys. J. 2012, 103, 1020–1027. [Google Scholar] [CrossRef]
- Hou, P.; Zhang, N.; Wu, R.; Xu, W.; Hou, Z. Photo-cross-linked biodegradable hydrogels based on narm-poly (ethylene glycol), poly (ε-caprolactone) and/or methacrylic acid for controlled drug release. J. Biomater. Appl. 2017, 32, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Yadav, I.; Purohit, S.D.; Singh, H.; Das, N.; Roy, P.; Mishra, N.C. A highly transparent tri-polymer complex in situ hydrogel of HA, collagen and four-arm-PEG as potential vitreous substitute. Biomed. Mater. 2021, 16, 065018. [Google Scholar] [CrossRef]
- Téllez-Soto, C.A.; Silva, M.G.P.; dos Santos, L.; Mendes, T.d.O.; Singh, P.; Fortes, S.A.; Favero, P.; Martin, A.A. In vivo determination of dermal water content in chronological skin aging by confocal Raman spectroscopy. Vib. Spectrosc. 2021, 112, 103196. [Google Scholar] [CrossRef]
- Borciani, G.; Montalbano, G.; Melo, P.; Baldini, N.; Ciapetti, G.; Vitale-Brovarone, C. Assessment of Collagen-Based Nanostructured Biomimetic Systems with a Co-Culture of Human Bone-Derived Cells. Cells 2021, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Kim, B.-S.; Gao, G. Advanced Strategies for 3D Bioprinting of Tissue and Organ Analogs Using Alginate Hydrogel Bioinks. Mar. Drugs 2021, 19, 708. [Google Scholar] [CrossRef] [PubMed]
- Román, J.; Cabañas, M.V.; Peña, J.; Vallet-Regí, M. Control of the pore architecture in three-dimensional hydroxyapatite-reinforced hydrogel scaffolds. Sci. Technol. Adv. Mater. 2011, 12, 045003. [Google Scholar] [CrossRef]
- Malhotra, D.; Pan, S.; Rüther, L.; Goudoulas, T.B.; Schlippe, G.; Voss, W.; Germann, N. Linear viscoelastic and microstructural properties of native male human skin and in vitro 3D reconstructed skin models. J. Mech. Behav. Biomed. Mater. 2019, 90, 644–654. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Q.; Kong, W.; Wang, J.; He, L.; Guo, L.; Lin, H.; Fan, H.; Fan, Y.; Zhang, X. Activated hyaluronic acid/collagen composite hydrogel with tunable physical properties and improved biological properties. Int. J. Biol. Macromol. 2020, 164, 2186–2196. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, L.; Zhou, J.; Mao, Z.; Gao, C.; Shen, J. Fabrication and physical and biological properties of fibrin gel derived from human plasma. Biomed. Mater. 2007, 3, 015001. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef]
- Melo, P.; Montalbano, G.; Fiorilli, S.; Vitale-Brovarone, C. 3D Printing in Alginic Acid Bath of In-Situ Crosslinked Collagen Composite Scaffolds. Materials 2021, 14, 6720. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Nadolna, K.; Owczarek, A. The physical and chemical properties of hydrogels based on natural polymers. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 151–172. [Google Scholar]
- Davidenko, N.; Campbell, J.; Thian, E.; Watson, C.; Cameron, R. Collagen–hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomater. 2010, 6, 3957–3968. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Kurdtabar, M. Collagen-based highly porous hydrogel without any porogen: Synthesis and characteristics. Eur. Polym. J. 2007, 43, 877–889. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Park, H.; Ha, D.-H.; Yun, H.-S.; Yi, H.-G.; Lee, H. 3D bioprinting of in vitro models using hydrogel-based bioinks. Polymers 2021, 13, 366. [Google Scholar] [CrossRef]
- Chircov, C.; Grumezescu, A.M.; Bejenaru, L.E. Hyaluronic acid-based scaffolds for tissue engineering. Romanian J. Morphol. Embryol. 2018, 59, 71–76. [Google Scholar]
- Bunyaratavej, P.; Wang, H.-L. Collagen Membranes: A Review. J. Periodontol. 2001, 72, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Song, H.J.; Lee, S.H.; Lee, C.K. Comparative study of various growth factors and cytokines on type I collagen and hyaluronan production in human dermal fibroblasts. J. Cosmet. Dermatol. 2014, 13, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.W.; Alt-Holland, A.; Egles, C.; Garlick, J.A. Three-Dimensional Tissue Models of Normal and Diseased Skin. Curr. Protoc. Cell Biol. 2008, 41, 19. [Google Scholar]
- Yin, Y.; Yang, Y.-J.; Xu, H. Hydrophobically modified hydrogels containing azoaromatic cross-links: Swelling properties, degradation in vivo and application in drug delivery. Eur. Polym. J. 2002, 38, 2305–2311. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ma, Z.; He, D.; Li, H. Modulating degradation of sodium alginate/bioglass hydrogel for improving tissue infiltration and promoting wound healing. Bioact. Mater. 2021, 6, 3692–3704. [Google Scholar] [CrossRef]
- Montalbano, G.; Borciani, G.; Pontremoli, C.; Ciapetti, G.; Mattioli-Belmonte, M.; Fiorilli, S.; Vitale-Brovarone, C. Development and Biocompatibility of Collagen-Based Composites Enriched with Nanoparticles of Strontium Containing Mesoporous Glass. Materials 2019, 12, 3719. [Google Scholar] [CrossRef]
- Sanami, M.; Sweeney, I.; Shtein, Z.; Meirovich, S.; Sorushanova, A.; Mullen, A.M.; Miraftab, M.; Shoseyov, O.; O’Dowd, C.; Pandit, A.; et al. The influence of poly (ethylene glycol) ether tetrasuccinimidyl glutarate on the structural, physical, and biological properties of collagen fibers. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Niamh, M.; Pandit, A.; Dowd, E. Encapsulation of primary dopaminergic neurons in a GDNF-loaded collagen hydrogel increases their survival, re-innervation and function after intra-striatal transplantation. Sci. Rep. 2017, 7, 16033. [Google Scholar]
- Greco, R.M.; Iocono, J.A.; Ehrlich, H.P. Hyaluronic acid stimulates human fibroblast proliferation within a collagen matrix. J. Cell. Physiol. 1998, 177, 465–473. [Google Scholar] [CrossRef]
- Xu, Q.; Torres, J.E.; Hakim, M.; Babiak, P.M.; Pal, P.; Battistoni, C.M.; Nguyen, M.; Panitch, A.; Solorio, L.; Liu, J.C. Collagen- and hyaluronic acid-based hydrogels and their biomedical applications. Mater. Sci. Eng. R Rep. 2021, 146. [Google Scholar] [CrossRef]
- Ahn, H.W.; Farmer, J.L.; Bazer, F.W.; Spencer, T.E. Progesterone and interferon tau-regulated genes in the ovine uterine endometrium: Identification of periostin as a potential mediator of conceptus elongation. Reproduction 2009, 138, 813–825. [Google Scholar] [CrossRef]
- Xie, H.; Chen, X.; Shen, X.; He, Y.; Chen, W.; Luo, Q.; Ge, W.; Yuan, W.; Tang, X.; Hou, D.; et al. Preparation of chitosan-collagen-alginate composite dressing and its promoting effects on wound healing. Int. J. Biol. Macromol. 2018, 107, 93–104. [Google Scholar] [CrossRef]
- Moura, L.I.; Dias, A.M.; Suesca, E.; Casadiegos, S.; Leal, E.C.; Fontanilla, M.R.; Carvalho, L.; de Sousa, H.C.; Carvalho, E. Neurotensin-loaded collagen dressings reduce inflammation and improve wound healing in diabetic mice. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 32–43. [Google Scholar] [CrossRef]
- Peattie, R.A.; Nayate, A.; Firpo, M.; Shelby, J.; Fisher, R.; Prestwich, G. Stimulation of in vivo angiogenesis by cytokine-loaded hyaluronic acid hydrogel implants. Biomaterials 2004, 25, 2789–2798. [Google Scholar] [CrossRef]
- Peattie, R.A.; Rieke, E.R.; Hewett, E.M.; Fisher, R.J.; Shu, X.Z.; Prestwich, G.D. Dual growth factor-induced angiogenesis in vivo using hyaluronan hydrogel implants. Biomaterials 2006, 27, 1868–1875. [Google Scholar] [CrossRef]
- Huang, J.; Ren, J.; Chen, G.; Li, Z.; Liu, Y.; Wang, G.; Wu, X. Tunable sequential drug delivery system based on chitosan/hyaluronic acid hydrogels and PLGA microspheres for management of non-healing infected wounds. Mater. Sci. Eng. C 2018, 89, 213–222. [Google Scholar] [CrossRef]
- Falanga, V.; Iwamoto, S.; Chartier, M.; Yufit, T.; Butmarc, J.; Kouttab, N.; Shrayer, D.; Carson, P. Autologous bone marrow–derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007, 13, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Breen, A.; O’Brien, T.; Pandit, A.; Gessmann, J.; Seybold, D.; Ayami, F.; Peter, E.; Baecker, H.; Schildhauer, T.A.; Koeller, M.; et al. Fibrin as a delivery system for therapeutic drugs and biomolecules. Tissue Eng. Part B Rev. 2009, 15, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Caiado, F.; Carvalho, T.; Silva, F.; Castro, C.; Clode, N.; Dye, J.F.; Dias, S. The role of fibrin E on the modulation of endothelial progenitors adhesion, differentiation and angiogenic growth factor production and the promotion of wound healing. Biomaterials 2011, 32, 7096–7105. [Google Scholar] [CrossRef] [PubMed]
- Longati, P.; Jia, X.; Eimer, J.; Wagman, A.; Witt, M.-R.; Rehnmark, S.; Verbeke, C.; Toftgård, R.; Löhr, M.; Heuchel, R.L. 3D pancreatic carcinoma spheroids induce a matrix-rich, chemoresistant phenotype offering a better model for drug testing. BMC Cancer 2013, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-Z.; Chang, H.-Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. Healthc. Nutr. Technol. 2008, 3, 1172–1184. [Google Scholar]
- Leung, B.M.; Lesher-Perez, S.C.; Matsuoka, T.; Moraes, C.; Takayama, S. Media additives to promote spheroid circularity and compactness in hanging drop platform. Biomater. Sci. 2014, 3, 336–344. [Google Scholar] [CrossRef]
- Azizipour, N.; Avazpour, R.; Weber, M.H.; Sawan, M.; Ajji, A.; Rosenzweig, D.H. Uniform Tumor Spheroids on Surface-Optimized Microfluidic Biochips for Reproducible Drug Screening and Personalized Medicine. Micromachines 2022, 13, 587. [Google Scholar] [CrossRef]
- Jiang, C.; Hsu, S.; Sun, Y.; Tsai, M. Quantitative Bioimage Analysis of Passaging Effect on the Migratory Behavior of Human Mesenchymal Stem Cells During Spheroid Formation. Cytom. Part A 2020, 97, 394–406. [Google Scholar] [CrossRef]
- Thakuri, P.S.; Gupta, M.; Plaster, M.; Tavana, H. Quantitative size-based analysis of tumor spheroids and responses to therapeutics. ASSAY Drug Dev. Technol. 2019, 17, 140–149. [Google Scholar] [CrossRef]
- Assmann, V.; Fieber, C.; Herrlich, P.; Hofmann, M.; Termeer, C.C.; Ahrens, T.; Simon, J.C. CD44 is the Principal Mediator of Hyaluronic-Acid-Induced Melanoma Cell Proliferation. J. Investig. Dermatol. 2001, 116, 93–101. [Google Scholar] [CrossRef]
- Dietrich, A.; Tanczos, E.; Vanscheidt, W.; Schöpf, E.; Simon, J. High CD44 surface expression on primary tumours of malignant melanoma correlates with increased metastatic risk and reduced survival. Eur. J. Cancer 1997, 33, 926–930. [Google Scholar] [CrossRef] [PubMed]









| Sample | Crosslinker |
|---|---|
| CHAF_1 | PEG (C/PEG 1:1) |
| CHAF_10 | PEG (C/PEG 1:10) |
| CHAF_50 | PEG (C/PEG 1:50) |
| Sample | G′ (kPa) | G″ (kPa) |
|---|---|---|
| CHAF_1 | 37.6 | 9.1 |
| CHAF_10 | 78.1 | 16.0 |
| CHAF_50 | 64.5 | 10.7 |
| Material Spectrum | Wavenumber (cm−1) | Chemical Bond |
|---|---|---|
| Collagen | 1657 | α-helix secondary structure [30] C=O stretching [31] |
| 1555 | N–H bending vibration [32] C–N stretching vibration [32] | |
| 1403 | C=O stretching of COO– [33] | |
| 1240 | C–N stretching [33] | |
| 1161, 1066 | CO stretching | |
| 860 | CH bending | |
| Hyaluronic Acid | 1738, 1617 | Amide I [34] |
| 1560 | Amide II [34] | |
| 1325 | Amide III [35] | |
| 2922 | C–H stretching | |
| 1082, 1043 | C–O, C–C, and C–OH groups [35] | |
| 944 | Carbohydrates [36] | |
| Fibrin | 1700–1600 | Amide I [37] |
| 1643, 1534 | Amide II [38,39] | |
| 1314 | Amide III | |
| 1077 | CO stretching [33] | |
| 4S-StarPEG | 2885 | –CH2- bonds [40] |
| 1100 | C–O–C bonds [40] | |
| 1740 | Succinimidyl ester C=O stretch | |
| 1467 | C–H stretching of methylene group of alkyl chain | |
| 1342, 1241 | Ester bonds | |
| 1147 | C–O–C stretching | |
| 1060 | C–O stretching | |
| 961, 843 | C–H bending [41] | |
| 1700–1600 | Amide I [37] | |
| 1557 | Amide II | |
| 1640 | NH–C=O (amide bond formation) | |
| 3320 | –OH groups | |
| 1366 | C=O stretching | |
| 1230 | Amide bond |
| Material | Water Content (%) |
|---|---|
| Human dermis | 70–75 |
| CHAF_1 | 71.8 ± 12.7 |
| Pore Size | ||
|---|---|---|
| Day 0 | Day 2 | Day 7 |
| 120 ± 97 μm | 131 ± 54 μm | 169 ± 82 μm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bindi, B.; Perioli, A.; Melo, P.; Mattu, C.; Ferreira, A.M. Bioinspired Collagen/Hyaluronic Acid/Fibrin-Based Hydrogels for Soft Tissue Engineering: Design, Synthesis, and In Vitro Characterization. J. Funct. Biomater. 2023, 14, 495. https://doi.org/10.3390/jfb14100495
Bindi B, Perioli A, Melo P, Mattu C, Ferreira AM. Bioinspired Collagen/Hyaluronic Acid/Fibrin-Based Hydrogels for Soft Tissue Engineering: Design, Synthesis, and In Vitro Characterization. Journal of Functional Biomaterials. 2023; 14(10):495. https://doi.org/10.3390/jfb14100495
Chicago/Turabian StyleBindi, Bianca, Annalisa Perioli, Priscila Melo, Clara Mattu, and Ana Marina Ferreira. 2023. "Bioinspired Collagen/Hyaluronic Acid/Fibrin-Based Hydrogels for Soft Tissue Engineering: Design, Synthesis, and In Vitro Characterization" Journal of Functional Biomaterials 14, no. 10: 495. https://doi.org/10.3390/jfb14100495
APA StyleBindi, B., Perioli, A., Melo, P., Mattu, C., & Ferreira, A. M. (2023). Bioinspired Collagen/Hyaluronic Acid/Fibrin-Based Hydrogels for Soft Tissue Engineering: Design, Synthesis, and In Vitro Characterization. Journal of Functional Biomaterials, 14(10), 495. https://doi.org/10.3390/jfb14100495