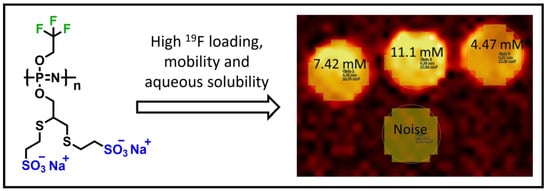
Superfluorinated, Highly Water-Soluble Polyphosphazenes as Potential 19F Magnetic Resonance Imaging (MRI) Contrast Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Poly(trifluoroethoxy-propargyloxy)phosphazene (TFE-Propargyl-PPz, P1)
2.2. Synthesis of Poly(trifluoroethoxy-propargyloxy-MESNa)phosphazene (TFE-MESNa-PPz, P2)
3. Results and Discussion
3.1. Polymer Synthesis and Characterization
3.2. Magnetic Resonance Imaging (MRI) and Spectroscopy (MRS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Runge, V.M. Notes on “Characteristics of Gadolinium-DTPA Complex: A Potential NMR Contrast Agent”. Am. J. Roentgenol. 2008, 190, 1433–1434. [Google Scholar] [CrossRef]
- Gries, H.; Rosenberg, D.; Weinmann, H.J. Paramagnetic complex salts, their preparation, and their use in NMR-diagnostics. ZA825313B, 25 May 1987. [Google Scholar]
- Weinmann, H.J.; Brasch, R.C.; Press, W.R.; Wesbey, G.E. Characteristics of gadolinium-DTPA complex: A potential NMR contrast agent. AJR Am. J. Roentgenol. 1984, 142, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Runge, V.M.; Clanton, J.A.; Price, A.C.; Herzer, W.A.; Allen, J.H.; Partain, C.L.; James, A.E.; Dyke Award, J.R. Evaluation of contrast-enhanced MR imaging in a brain-abscess model. AJNR Am. J. Neuroradiol. 1985, 6, 139. [Google Scholar] [PubMed]
- Bui-Mansfield, L.T. Top 100 Cited AJR Articles at the AJR’s Centennial. Am. J. Roentgenol. 2006, 186, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Laniado, M.; Weinmann, H.J.; Schörner, W.; Felix, R.; Speck, U. First use of GdDTPA/dimeglumine in man. Physiol. Chem. Phys. Med. NMR 1984, 16, 157–165. [Google Scholar] [PubMed]
- Weinmann, H.J.; Laniado, M.; Mützel, W. Pharmacokinetics of GdDTPA/dimeglumine after intravenous injection into healthy volunteers. Physiol. Chem. Phys. Med. NMR 1984, 16, 167–172. [Google Scholar] [PubMed]
- Lohrke, J.; Frenzel, T.; Endrikat, J.; Alves, F.C.; Grist, T.M.; Law, M.; Lee, J.M.; Leiner, T.; Li, K.-C.; Nikolaou, K.; et al. 25 Years of Contrast-Enhanced MRI: Developments, Current Challenges and Future Perspectives. Adv. Ther. 2016, 33, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Schieda, N.; Blaichman, J.I.; Costa, A.F.; Glikstein, R.; Hurrell, C.; James, M.; Jabehdar Maralani, P.; Shabana, W.; Tang, A.; Tsampalieros, A.; et al. Gadolinium-Based Contrast Agents in Kidney Disease: A Comprehensive Review and Clinical Practice Guideline Issued by the Canadian Association of Radiologists. Can. J. Kidney Health Dis. 2018, 5, 136–150. [Google Scholar] [CrossRef]
- Iyad, N.; Ahmad, M.S.; Alkhatib, S.G.; Hjouj, M. Gadolinium contrast agents- challenges and opportunities of a multidisciplinary approach: Literature review. Eur. J. Radiol. Open 2023, 11, 100503. [Google Scholar] [CrossRef]
- Runge, V.M. Critical Questions Regarding Gadolinium Deposition in the Brain and Body After Injections of the Gadolinium-Based Contrast Agents, Safety, and Clinical Recommendations in Consideration of the EMA’s Pharmacovigilance and Risk Assessment Committee Recommendation for Suspension of the Marketing Authorizations for 4 Linear Agents. Investig. Radiol. 2017, 52, 317–323. [Google Scholar] [CrossRef]
- Mo, Y.; Huang, C.; Liu, C.; Duan, Z.; Liu, J.; Wu, D. Recent Research Progress of 19 F Magnetic Resonance Imaging Probes: Principle, Design, and Their Application. Macromol. Rapid Commun. 2023, 44, 2200744. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, A.H.; Caruthers, S.D.; Keupp, J.; Wickline, S.A.; Lanza, G.M. Recent Advances in 19Fluorine Magnetic Resonance Imaging with Perfluorocarbon Emulsions. Engineering 2015, 1, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Winter, P.M. Perfluorocarbon Nanoparticles: Evolution of a Multimodality and Multifunctional Imaging Agent. Scientifica 2014, 2014, 746574. [Google Scholar] [CrossRef] [PubMed]
- Chapelin, F.; Gedaly, R.; Sweeney, Z.; Gossett, L.J. Prognostic Value of Fluorine-19 MRI Oximetry Monitoring in cancer. Mol. Imaging Biol. 2022, 24, 208–219. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, R.; Liu, F.; Ping, J.; Wen, X.; Wang, H.; Wang, K.; Sun, X.; Zou, H.; Shen, B.; et al. 19 F MRI in orthotopic cancer model via intratracheal administration of α ν β 3 -targeted perfluorocarbon nanoparticles. Nanomedicine 2018, 13, 2551–2562. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Vanherwegen, A.-S.; Liang, S.; Verbeke, R.; Korf, H.; Lentacker, I.; de Smedt, S.C.; Gysemans, C.; Himmelreich, U. Fluorine MR Imaging Probes Dynamic Migratory Profiles of Perfluorocarbon-Loaded Dendritic Cells After Streptozotocin-Induced Inflammation. Mol. Imaging Biol. 2022, 24, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Tennie, I.K.; Kilbinger, A.F.M. Polymeric 19 F MRI Contrast Agents Prepared by Ring-Opening Metathesis Polymerization/Dihydroxylation. Macromolecules 2020, 53, 10386–10396. [Google Scholar] [CrossRef]
- Fu, C.; Demir, B.; Alcantara, S.; Kumar, V.; Han, F.; Kelly, H.G.; Tan, X.; Yu, Y.; Xu, W.; Zhao, J.; et al. Low-Fouling Fluoropolymers for Bioconjugation and In Vivo Tracking. Angew. Chem. Int. Ed. 2020, 59, 4729–4735. [Google Scholar] [CrossRef]
- Taylor, N.G.; Chung, S.H.; Kwansa, A.L.; Johnson, R.R.; Teator, A.J.; Milliken, N.J.B.; Koshlap, K.M.; Yingling, Y.G.; Lee, Y.Z.; Leibfarth, F.A. Partially Fluorinated Copolymers as Oxygen Sensitive 19 F MRI Agents. Chem. A Eur. J. 2020, 26, 9982–9990. [Google Scholar] [CrossRef]
- Rothemund, S.; Teasdale, I. Preparation of polyphosphazenes: A tutorial review. Chem. Soc. Rev. 2016, 5200, 5200–5215. [Google Scholar] [CrossRef] [PubMed]
- Allcock, H.R. The expanding field of polyphosphazene high polymers. Dalton Trans. 2016, 45, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Strasser, P.; Teasdale, I. Main-Chain Phosphorus-Containing Polymers for Therapeutic Applications. Molecules 2020, 25, 1716. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.K. Polyphosphazenes as Vaccine Adjuvants. In Vaccine Adjuvants and Delivery Systems; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 355–378. [Google Scholar]
- Wilfert, S.; Henke, H.; Schoefberger, W.; Brüggemann, O.; Teasdale, I. Chain-End-Functionalized Polyphosphazenes via a One-Pot Phosphine-Mediated Living Polymerization. Macromol. Rapid Commun. 2014, 35, 1135–1141. [Google Scholar] [CrossRef]
- Strasser, P.; Plavcan, O.; Ajvazi, E.; Henke, H.; Brüggemann, O.; Teasdale, I. Hetero and homo α,ω-chain-end functionalized polyphosphazenes. J. Polym. Sci. 2022, 60, 2000–2007. [Google Scholar] [CrossRef] [PubMed]
- Bates, M.C.; Yousaf, A.; Sun, L.; Barakat, M.; Kueller, A. Translational Research and Early Favorable Clinical Results of a Novel Polyphosphazene (Polyzene-F) Nanocoating. Regen. Eng. Transl. Med. 2019, 5, 341–353. [Google Scholar] [CrossRef]
- Sharma, R.; Singla, N.; Mehta, S.; Gaba, T.; Rawal, R.; Rao, H.S.; Bhardwaj, T.R. Recent Advances in Polymer Drug Conjugates. MRMC 2015, 15, 751–761. [Google Scholar] [CrossRef]
- Lin, M.; Marin, A.; Ellis, B.; Eubanks, L.M.; Andrianov, A.K.; Janda, K.D. Polyphosphazene: A New Adjuvant Platform for Cocaine Vaccine Development. Mol. Pharm. 2022, 19, 3358–3366. [Google Scholar] [CrossRef]
- Strasser, P.; Montsch, B.; Weiss, S.; Sami, H.; Kugler, C.; Hager, S.; Schueffl, H.; Mader, R.; Brüggemann, O.; Kowol, C.R.; et al. Degradable Bottlebrush Polypeptides and the Impact of their Architecture on Cell Uptake, Pharmacokinetics, and Biodistribution In Vivo. Small 2023, 19, 2300767. [Google Scholar] [CrossRef]
- Wang, B.; Rivard, E.; Manners, I. A New High-Yield Synthesis of Cl3PNSiMe3, a Monomeric Precursor for the Controlled Preparation of High Molecular Weight Polyphosphazenes. Inorg. Chem. 2002, 41, 1690–1691. [Google Scholar] [CrossRef]
- Li, Z.; Chen, C.; McCaffrey, M.; Yang, H.; Allcock, H.R. Polyphosphazene Elastomers with Alkoxy and Trifluoroethoxy Side Groups. ACS Appl. Polym. Mater. 2020, 2, 475–480. [Google Scholar] [CrossRef]
- Okaru, A.O.; Brunner, T.S.; Ackermann, S.M.; Kuballa, T.; Walch, S.G.; Kohl-Himmelseher, M.; Lachenmeier, D.W. Application of 19 F NMR Spectroscopy for Content Determination of Fluorinated Pharmaceuticals. J. Anal. Methods Chem. 2017, 2017, 9206297. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, M.; Bauer, H.; Mintorovitch, J.; Requardt, M.; Weinmann, H.-J. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Investig. Radiol. 2005, 40, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Goetschi, S.; Froehlich, J.M.; Chuck, N.C.; Curcio, R.; Runge, V.M.; Andreisek, G.; Nanz, D.; Boss, A. The protein and contrast agent-specific influence of pathological plasma-protein concentration levels on contrast-enhanced magnetic resonance imaging. Investig. Radiol. 2014, 49, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Tirotta, I.; Mastropietro, A.; Cordiglieri, C.; Gazzera, L.; Baggi, F.; Baselli, G.; Bruzzone, M.G.; Zucca, I.; Cavallo, G.; Terraneo, G.; et al. A Superfluorinated Molecular Probe for Highly Sensitive in Vivo19 F-MRI. J. Am. Chem. Soc. 2014, 136, 8524–8527. [Google Scholar] [CrossRef] [PubMed]
- Mastropietro, A.; de Bernardi, E.; Breschi, G.L.; Zucca, I.; Cametti, M.; Soffientini, C.D.; de Curtis, M.; Terraneo, G.; Metrangolo, P.; Spreafico, R.; et al. Optimization of rapid acquisition with relaxation enhancement (RARE) pulse sequence parameters for ¹⁹F-MRI studies. J. Magn. Reson. Imaging 2014, 40, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Colotti, R.; Bastiaansen, J.A.M.; Wilson, A.; Flögel, U.; Gonzales, C.; Schwitter, J.; Stuber, M.; van Heeswijk, R.B. Characterization of perfluorocarbon relaxation times and their influence on the optimization of fluorine-19 MRI at 3 tesla. Magn. Reson. Med. 2017, 77, 2263–2271. [Google Scholar] [CrossRef]
- Jirak, D.; Galisova, A.; Kolouchova, K.; Babuka, D.; Hruby, M. Fluorine polymer probes for magnetic resonance imaging: Quo vadis? Magn. Reson. Mater. Phy. 2019, 32, 173–185. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strasser, P.; Schinegger, V.; Friske, J.; Brüggemann, O.; Helbich, T.H.; Teasdale, I.; Pashkunova-Martic, I. Superfluorinated, Highly Water-Soluble Polyphosphazenes as Potential 19F Magnetic Resonance Imaging (MRI) Contrast Agents. J. Funct. Biomater. 2024, 15, 40. https://doi.org/10.3390/jfb15020040
Strasser P, Schinegger V, Friske J, Brüggemann O, Helbich TH, Teasdale I, Pashkunova-Martic I. Superfluorinated, Highly Water-Soluble Polyphosphazenes as Potential 19F Magnetic Resonance Imaging (MRI) Contrast Agents. Journal of Functional Biomaterials. 2024; 15(2):40. https://doi.org/10.3390/jfb15020040
Chicago/Turabian StyleStrasser, Paul, Verena Schinegger, Joachim Friske, Oliver Brüggemann, Thomas H. Helbich, Ian Teasdale, and Irena Pashkunova-Martic. 2024. "Superfluorinated, Highly Water-Soluble Polyphosphazenes as Potential 19F Magnetic Resonance Imaging (MRI) Contrast Agents" Journal of Functional Biomaterials 15, no. 2: 40. https://doi.org/10.3390/jfb15020040
APA StyleStrasser, P., Schinegger, V., Friske, J., Brüggemann, O., Helbich, T. H., Teasdale, I., & Pashkunova-Martic, I. (2024). Superfluorinated, Highly Water-Soluble Polyphosphazenes as Potential 19F Magnetic Resonance Imaging (MRI) Contrast Agents. Journal of Functional Biomaterials, 15(2), 40. https://doi.org/10.3390/jfb15020040