Genipin-Enhanced Fibrin Hydrogel and Novel Silk for Intervertebral Disc Repair in a Loaded Bovine Organ Culture Model
Abstract
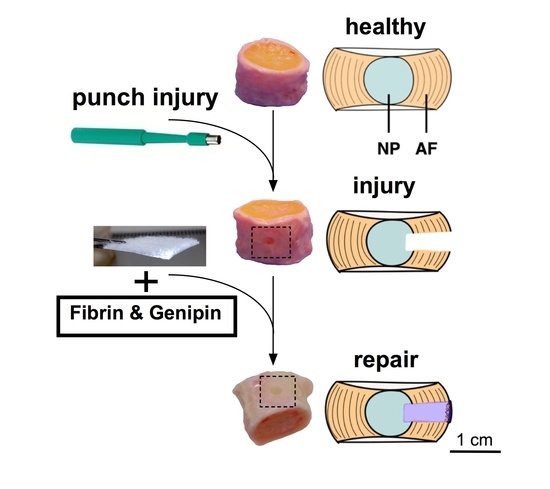
:1. Introduction
2. Results
2.1. Stress-Strain Measurement
2.2. Cytotoxicity of Genipin
2.3. Organ Culture
2.4. Gene Expression
2.5. Histology
3. Discussion
3.1. IVD Injury versus Repair
3.2. Toxicity of Genipin
3.3. Limitations and Strengths
- A constructed GMP-compliant silk-fleece material was able to seal the outer AF and stayed in place over 14 days of repetitive mechanical loading using a fibrin hydrogel as a filler material.
- Cytotoxic assays of genipin in monolayer assays demonstrated a high cytotoxicity, representing hurdles for the American Food and Drug Administration (FDA) or the Conformité Européene (CE) approval for future research towards clinical application.
- The ex vivo organ culture model to test the effect of biomaterials onto the cell viability/cell activity was limited to 14 days due to the chosen bovine model with endplates attached, which limited the diffusion of glucose or other unknown factors over longer culture periods. Here, application of improved preparation techniques and culture media might allow to prolong the culture period and mechanical loading [32].
3.4. Future Road Map for Fine-Tuning Hydrogels and/or Silk
4. Material and Methods
4.1. IVD Isolation and Culture
4.2. Injury and Repair Model
4.3. Stress–Strain Measurement
4.4. Cytotoxicity of Genipin
4.5. Mitochondrial Activity
4.6. DNA Content
4.7. Extracellular Matrix Content
4.8. Relative Gene Expression
4.9. Histology
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Frauchiger, D.A.; Tekari, A.; Wöltje, M.; Fortunato, G.; Benneker, L.M.; Gantenbein, B. A review of the application of reinforced hydrogels and silk as biomaterials for intervertebral disc repair. Eur. Cells Mater. 2017, 34, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Pennicooke, B.; Hussain, I.; Berlin, C.; Sloan, S.R.; Borde, B.; Moriguchi, Y.; Lang, G.; Navarro-Ramirez, R.; Cheetham, J.; Bonassar, L.J.; et al. Annulus Fibrosus Repair Using High-Density Collagen Gel: An In vivo Ovine Model. Spine 2017, 43, E208–E215. [Google Scholar] [CrossRef] [PubMed]
- Elliott, W.H.; Bonani, W.; Maniglio, D.; Motta, A.; Tan, W.; Migliaresi, C. Silk Hydrogels of Tunable Structure and Viscoelastic Properties Using Different Chronological Orders of Genipin and Physical Cross-Linking. ACS Appl. Mater. Interfaces 2015, 7, 12099–12108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, R.G.; Bürki, A.; Zysset, P.; Eglin, D.; Grijpma, D.W.; Blanquer, S.B.; Hecht, A.C.; Iatridis, J.C. Mechanical restoration and failure analyses of a hydrogel and scaffold composite strategy for annulus fibrosus repair. Acta Biomater. 2016, 30, 116–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Likhitpanichkul, M.; Dreischarf, M.; Illien-Junger, S.; Walter, B.A.; Nukaga, T.; Long, R.G.; Sakai, D.; Hecht, A.C.; Iatridis, J.C. Fibrin-genipin adhesive hydrogel for annulus fibrosus repair: Performance evaluation with large animal organ culture, in situ biomechanics, and in vivo degradation tests. Eur. Cells Mater. 2014, 28, 25–38. [Google Scholar] [CrossRef]
- Likhitpanichkul, M.; Kim, Y.; Torre, O.M.; See, E.; Kazezian, Z.; Pandit, A.; Hecht, A.C.; Iatridis, J.C. Fibrin-genipin annulus fibrosus sealant as a delivery system for anti-TNFα drug. Spine J. 2015, 15, 2045–2054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Liu, H.; Wong, E.J.; Toh, S.L.; Goh, J.C. In vivo study of anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold. Biomaterials 2008, 29, 3324–3337. [Google Scholar] [CrossRef] [PubMed]
- Frauchiger, D.A.; Heeb, S.R.; May, R.D.; Wöltje, M.; Benneker, L.M.; Gantenbein, B. Differentiation of MSC and annulus fibrosus cells on genetically-engineered silk fleece-membrane-composites enriched for GDF-6 or TGF-β3. J. Orthop. Res. 2017, 36, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, B.; Guo, F.; Du, J.; Gu, P.; Lin, X.; Yang, W.; Zhang, H.; Lu, M.; Huang, Y.; et al. Injectable silk fibroin/polyurethane composite hydrogel for nucleus pulposus replacement. J. Mater. Sci. Mater. Med. 2012, 23, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Kim, H.W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 2017, 63, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, H.; Sun, L.; Cairns, D.M.; Rainbow, R.S.; Preda, R.C.; Kaplan, D.L.; Zeng, L. The influence of scaffold material on chondrocytes under inflammatory conditions. Acta Biomater. 2013, 9, 6563–6575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arasteh, S.; Kazemnejad, S.; Khanjani, S.; Heidari-Vala, H.; Akhondi, M.M.; Mobini, S. Fabrication and characterization of nano-fibrous bilayer composite for skin regeneration application. Methods 2016, 99, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Pramanik, K. Development of novel silk fibroin/polyvinyl alcohol/sol-gel bioactive glass composite matrix by modified layer by layer electrospinning method for bone tissue construct generation. Biofabrication 2017, 9, 015028. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.A.; McAnany, S.; Gupta, N.; Long, R.; Nasser, P.R.; Eglin, D.; Hecht, A.; Illien-Junger, S.; Iatridis, J.C. Structural and Chemical Modification to Improve Adhesive and Material Properties of Fibrin-Genipin for Repair of Annulus Fibrosus Defects in Intervertebral Discs. J. Biomech. Eng. 2017, 139. [Google Scholar] [CrossRef] [PubMed]
- Gantenbein, B.; Illien-Jünger, S.; Chan, S.C.; Walser, J.; Haglund, L.; Ferguson, S.J.; Iatridis, J.C.; Grad, S. Organ Culture Bioreactors—Platforms to Study Human Intervertebral Disc Degeneration and Regenerative Therapy. Curr. Stem Cells Res. Ther. 2015, 10, 339–352. [Google Scholar] [CrossRef]
- Paul, C.P.; Zuiderbaan, H.A.; Zandieh Doulabi, B.; van der Veen, A.J.; van de Ven, P.M.; Smit, T.H.; Helder, M.N.; van Royen, B.J.; Mullender, M.G. Simulated-physiological loading conditions preserve biological and mechanical properties of caprine lumbar intervertebral discs in ex vivo culture. PLoS ONE 2012, 7, e33147. [Google Scholar] [CrossRef] [PubMed]
- Walter, B.A.; Illien-Jünger, S.; Nasser, P.R.; Hecht, A.C.; Iatridis, J.C. Development and validation of a bioreactor system for dynamic loading and mechanical characterization of whole human intervertebral discs in organ culture. J. Biomech. 2014, 47, 2095–2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraysse, F.; Costi, J.J.; Stanley, R.M.; Ding, B.; McGuire, D.; Eng, K.; Bain, G.I.; Thewlis, D. A novel method to replicate the kinematics of the carpus using a six degree-of-freedom robot. J. Biomech. 2014, 47, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Haglund, L.; Moir, J.; Beckman, L.; Mulligan, K.R.; Jim, B.; Ouellet, J.A.; Roughley, P.; Steffen, T. Development of a Bioreactor for Axially Loaded Intervertebral Disc Organ Culture. Tissue Eng. Part C Methods 2011, 17, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Walser, J.; Gantenbein-Ritter, B. Design of a Mechanical Loading Device to Culture Intact Bovine Caudal Motional Segments of the Spine under Twisting Motion. In Replacing Animal Models: A Practical Guide to Creating and Using Biomimetic Alternatives; Davies, J., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2012; pp. 89–105. ISBN 9781119940685. [Google Scholar]
- Freemont, A.J.; Watkins, A.; Le Maitre, C.; Baird, P.; Jeziorska, M.; Knight, M.T.; Ross, E.R.; O’Brien, J.P.; Hoyland, J.A. Nerve growth factor expression and innervation of the painful intervertebral disc. J. Pathol. 2002, 197, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.Z.; Daly, C.D.; Ghosh, P.; Jenkin, G.; Oehme, D.; Cooper-White, J.; Naidoo, T.; Goldschlager, T. Ovine Lumbar Intervertebral Disc Degeneration Model Utilizing a Lateral Retroperitoneal Drill Bit Injury. J. Vis. Exp. 2017, 123. [Google Scholar] [CrossRef] [PubMed]
- Guterl, C.C.; Torre, O.M.; Purmessur, D.; Dave, K.; Likhitpanichkul, M.; Hecht, A.; Nicoll, S.; Iatridis, J.C. Characterization of Mechanics and Cytocompatibility of Fibrin-Genipin Annulus Fibrosus Sealant with the Addition of Cell Adhesion Molecules. Tissue Eng. Part A 2014, 20, 2536–2545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matcham, S.; Novakovic, K. Fluorescence Imaging in Genipin Crosslinked Chitosan-Poly (vinyl pyrrolidone) Hydrogels. Polymers 2016, 8, 385. [Google Scholar] [CrossRef]
- Chan, S.C.W.; Walser, J.; Käppeli, P.; Shamsollahi, M.J.; Ferguson, S.J.; Gantenbein-Ritter, B. Region Specific Response of Intervertebral Disc Cells to Complex Dynamic Loading: An Organ Culture Study Using a Dynamic Torsion-Compression Bioreactor. PLoS ONE 2013, 8, e72489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schek, R.M.; Michalek, A.J.; Iatridis, J.C. Genipin-crosslinked fibrin hydrogels as a potential adhesive to augment intervertebral disc annulus repair. Eur. Cells Mater. 2011, 21, 373–383. [Google Scholar] [CrossRef]
- Grant, M.; Epure, L.M.; Salem, O.; AlGarni, N.; Ciobanu, O.; Alaqeel, M.; Antoniou, J.; Mwale, F. Development of a Large Animal Long-Term Intervertebral Disc Organ Culture Model That Includes the Bony Vertebrae for Ex Vivo Studies. Tissue Eng. Part C Methods 2016, 22, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Demers, C.N.; Antoniou, J.; Mwale, F. Value and limitations of using the bovine tail as a model for the human lumbar spine. Spine 2004, 29, 2793–2799. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lezuo, P.; Pattappa, G.; Collin, E.; Alini, M.; Grad, S.; Peroglio, M. Development of an ex vivo cavity model to study repair strategies in loaded intervertebral discs. Eur. Spine J. 2016, 25, 2898–2908. [Google Scholar] [CrossRef] [PubMed]
- Frauchiger, D.A.; Chan, S.C.W.; Benneker, L.M.; Gantenbein, B. Intervertebral disc damage models in organ culture: A comparison of annulus fibrosus cross-incision versus punch model under complex loading. Eur. Spine J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, B.; Rosenzweig, D.H.; Krock, E.; Roughley, P.J.; Beckman, L.; Steffen, T.; Weber, M.H.; Ouellet, J.A.; Haglund, L. Acute mechanical injury of the human intervertebral disc: Link to degeneration and pain. Eur. Cells Mater. 2014, 28, 98–110. [Google Scholar] [CrossRef]
- Guterl, C.C.; See, E.Y.; Blanquer, S.B.; Pandit, A.; Ferguson, S.J.; Benneker, L.M.; Grijpma, D.W.; Sakai, D.; Eglin, D.; Alini, M.; et al. Challenges and strategies in the repair of ruptured annulus fibrosus. Eur. Cells Mater. 2013, 25, 1–21. [Google Scholar] [CrossRef]
- Adams, M.A.; Roughley, P.J. What is intervertebral disc degeneration, and what causes it? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.C.; Gantenbein-Ritter, B. Preparation of intact bovine tail intervertebral discs for organ culture. J. Vis. Exp. 2012, 60, e3490. [Google Scholar] [CrossRef] [PubMed]
- Kupcsik, L.; Alini, M.; Stoddart, M.J. Epsilon-aminocaproic acid is a useful fibrin degradation inhibitor for cartilage tissue engineering. Tissue Eng. Part A 2009, 15, 2309–2313. [Google Scholar] [CrossRef] [PubMed]
- Guggisberg, S.; Benneker, L.M.; Keel, M.J.; Gantenbein, B. Mechanical Loading Promoted Discogenic Differentiation of Human Mesenchymal Stem Cells Incorporated in 3D-PEG Scaffolds with RhGDF5 and RGD. Int. J. Stem Cells Res. Ther. 2015, 2, 006. [Google Scholar] [CrossRef]
- Gantenbein-Ritter, B.; Benneker, L.M.; Alini, M.; Grad, S. Differential response of human bone marrow stromal cells to either TGF-β(1) or rhGDF-5. Eur. Spine J. 2011, 20, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Farndale, R.W.; Buttle, D.J.; Barrett, A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta 1986, 883, 173–177. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Alini, M.; Mauck, R.; Sakai, D. With or without cells, that is the question! JOR Spine 2018, 1, e1010. [Google Scholar] [CrossRef] [Green Version]








| Cell Type | Gender | Age | Location | Passage |
|---|---|---|---|---|
| hMSC | Female | 62 | T1/2 | 3 |
| hMSC | Male | 86 | L1/2 | 2 |
| hMSC | Female | 75 | L3 | 3 |
| hMSC | Female | 60 | L1-3 | 2 |
| hMSC | Male | 72 | T8 | 2 |
| hMSC | Female | 69 | T3-8 | 1 |
| hAF & hNP | Male | 47 | T12/l1 | 2 |
| hAF & hNP | Male | 50 | L2/3 | 2 |
| hAF & hNP | Female | 38 | L5/6 | 2 |
| Medium | Abbreviation | DMSO (%) | Genipin (%) |
|---|---|---|---|
| Control | C | - | |
| DMSO 1 | D1 | 1.45 | - |
| DMSO 2 | D2 | 2.91 | - |
| DMSO 3 | D3 | 6.5 | - |
| DMSO 4 | D4 | 8.72 | - |
| DMSO 5 | D5 | 11.63 | - |
| Genipin 1 | G1 | 1.45 | 0.1 |
| Genipin 2 | G2 | 2.91 | 0.2 |
| Genipin 3 | G3 | 6.5 | 0.42 |
| Genipin 4 | G4 | 8.72 | 0.6 |
| Genipin 5 | G5 | 11.63 | 0.8 |
| Gene | Description | Forward Primer (5′-3′) | Reverse Primer (3′-5′) |
|---|---|---|---|
| 18S | 18S ribosomal RNA | ACG GAC AGG ATT GAC AGA TTG | CCA GAG TCT CGT TCG TTA TCG |
| ACAN | Aggrecan | GGC ATC GTG TTC CAT TAC AG | ACT CGT CCT TGT CTC CAT AG |
| COL1 | Collagen type I alpha 2 chain | GCC TCG CTC ACC AAC TTC | AGT AAC CAC TGC TCC ATT CTG |
| COL2 | Collagen type II alpha 1 chain | CGG GTG AAC GTG GAG AGA CA | GTC CAG GGT TGC CAT TGG AG |
| BGN | Biglycan | CTG CCA CTG CCA TCT GAG | TTG TTC ACG AGG ACC AAG G |
| COMP | Cartilage oligomeric matrix protein | TGC GAC GACGAC ATA CAC | ATC TCC TAC ACC ATC ACC ATC |
| MMP3 | Matrix metallopeptidase 3 | CTT CCG ATT CTG CTG TTG CTA TG | ATG GTG TCT TCC TTG TCC CTT G |
| MMP13 | Matrix metallopeptidase 13 | TCC TGG CTG GCT TCC TCT TC | CCT CGG ACA AGT CTT CAG AAT CTC |
| ADAMTS4 | ADAM metallopeptidase with thrombospondin type 1 motif 4 | GGC ACT GGG CTA CTA TTA C | TGG ACA CAG ACT GAG GAG |
| IL-1β | Interleukin 1 beta | AGT GCC ATC CTT CTG TCA | CAT TGC CTT CTC CGC TAT T |
| IL-8 | Interleukin 8 | CTT GTT CAA TAT GAC TTC CA | CCA CTC TCA ATA ACT CTC A |
| CCL2 | Chemokine (C-C motif) ligand 2 | TCG CCT GCT GCT ATA CAT T | TTG CTG CTG GTG ACT CTT |
| COX2 | Cytochrome c oxidase subunit II | GGT AAT CCT ATA TGC TCT C | GTA TCT TGA ACA CTG AAT G |
| NGF | Nerve growth factor | ATG TTG TTC TAC ACT CTG | ATG CTG AAG TTT AAT CCA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frauchiger, D.A.; May, R.D.; Bakirci, E.; Tekari, A.; Chan, S.C.W.; Wöltje, M.; Benneker, L.M.; Gantenbein, B. Genipin-Enhanced Fibrin Hydrogel and Novel Silk for Intervertebral Disc Repair in a Loaded Bovine Organ Culture Model. J. Funct. Biomater. 2018, 9, 40. https://doi.org/10.3390/jfb9030040
Frauchiger DA, May RD, Bakirci E, Tekari A, Chan SCW, Wöltje M, Benneker LM, Gantenbein B. Genipin-Enhanced Fibrin Hydrogel and Novel Silk for Intervertebral Disc Repair in a Loaded Bovine Organ Culture Model. Journal of Functional Biomaterials. 2018; 9(3):40. https://doi.org/10.3390/jfb9030040
Chicago/Turabian StyleFrauchiger, Daniela A., Rahel D. May, Ezgi Bakirci, Adel Tekari, Samantha C. W. Chan, Michael Wöltje, Lorin M. Benneker, and Benjamin Gantenbein. 2018. "Genipin-Enhanced Fibrin Hydrogel and Novel Silk for Intervertebral Disc Repair in a Loaded Bovine Organ Culture Model" Journal of Functional Biomaterials 9, no. 3: 40. https://doi.org/10.3390/jfb9030040
APA StyleFrauchiger, D. A., May, R. D., Bakirci, E., Tekari, A., Chan, S. C. W., Wöltje, M., Benneker, L. M., & Gantenbein, B. (2018). Genipin-Enhanced Fibrin Hydrogel and Novel Silk for Intervertebral Disc Repair in a Loaded Bovine Organ Culture Model. Journal of Functional Biomaterials, 9(3), 40. https://doi.org/10.3390/jfb9030040