A Decellularized Porcine Xenograft-Derived Bone Scaffold for Clinical Use as a Bone Graft Substitute: A Critical Evaluation of Processing and Structure
Abstract
:1. Introduction
2. Results
2.1. Structural Imaging
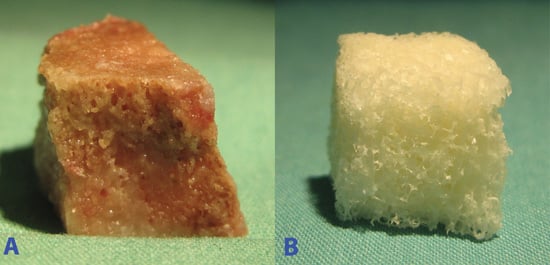
2.1.1. Macroscopic Field Images
2.1.2. Scanning Electron Microscopy
2.1.3. Micro-CT Imaging
2.2. Assessment of Decellularization
2.2.1. Histology
2.2.2. Measurement of DNA Content
2.3. Structural Characterization
2.3.1. Ultrastructure Measurements
2.3.2. Finite Element Analysis (FEA)
2.3.3. Mechanical Compression Testing
2.4. Proteomics
3. Discussion
4. Materials and Methods
4.1. Bone Scaffold Production
4.1.1. Acquisition of Animal Tissue
4.1.2. Physical Processing of Specimens
4.1.3. Decellularization and Chemical Oxidation Protocol
4.2. Structural Imaging
4.2.1. Scanning Electron Microscopy
4.2.2. Micro Computed Tomography
4.3. Assessment of Decellularization
4.3.1. Histology
4.3.2. DNA Quantification
4.4. Structural Characterization
4.4.1. Density, Porosity, Microstructure
4.4.2. Finite Element Analysis
4.4.3. Mechanical Compression Testing
4.5. Proteomics
Mass Spectrometry
4.6. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Drosse, I.; Volkmer, E.; Capanna, R.; De Biase, P.; Mutschler, W.; Schieker, M. Tissue engineering for bone defect healing: An update on a multi-component approach. Injury 2008, 39 (Suppl. 2), S9–S20. [Google Scholar] [CrossRef]
- Sela, J.J.; Bab, I.A. Part I: Physiology of bone healing. In Principles of Bone Regeneration; Sela, J.J., Bab, I.A., Eds.; Springer: New York, NY, USA, 2012; pp. 10–48. [Google Scholar]
- Calori, G.M.; Mazza, E.; Colombo, M.; Ripamonti, C. The use of bone-graft substitutes in large bone defects: Any specific needs? Injury 2011, 42 (Suppl. 2), S56–S63. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3d biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Gruskin, E.; Doll, B.A.; Futrell, F.W.; Schmitz, J.P.; Hollinger, J.O. Demineralized bone matrix in bone repair: History and use. Adv. Drug Deliv. Rev. 2012, 64, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Mauffrey, C.; Barlow, B.T.; Smith, W. Management of segmental bone defects. J. Am. Acad. Orthop. Surg. 2015, 23, 143–153. [Google Scholar] [PubMed]
- Laurencin, C.T.; El-Amin, S.F. Xenotransplantation in orthopaedic surgery. J. Am. Acad. Orthop. Surg. 2008, 16, 4–8. [Google Scholar] [CrossRef]
- Bansal, M.R.; Bhagat, S.B.; Shukla, D.D. Bovine cancellous xenograft in the treatment of tibial plateau fractures in elderly patients. Int. Orthop. 2009, 33, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Elliot, R.R.; Richards, R.H. Failed operative treatment in two cases of pseudarthrosis of the clavicle using internal fixation and bovine cancellous xenograft (tutobone). J. Pediatr. Orthop. Part B 2011, 20, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Ledford, C.K.; Nunley, J.A., 2nd; Viens, N.A.; Lark, R.K. Bovine xenograft failures in pediatric foot reconstructive surgery. J. Pediatr. Orthop. 2013, 33, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Loppini, M.; Longo, U.G.; Denaro, V.; Oliva, F. Bovine xenograft locking puddu plate versus tricalcium phosphate spacer non-locking puddu plate in opening-wedge high tibial osteotomy: A prospective double-cohort study. Int. Orthop. 2013, 37, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Auyeung, J.; Gower, A. Outcome of subtalar fusion using bovine cancellous bone graft: A retrospective case series. J. Foot Ankle Surg. 2011, 50, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Jupiter, D.C.; Clawson, L.D.; La Fontaine, J. Incorporation of bovine-based structural bone grafts used in reconstructive foot surgery. J. Foot Ankle Surg. 2012, 51, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Fu, L.; Liu, J.; Li, D. The expression and distribution of xenogeneic targeted antigens on porcine bone tissue. Transp. Proc. 2012, 44, 1419–1422. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Lian, Y.; Zhou, Z.; Lu, Y.; Li, S.; Pei, F.; Cheng, J. Distribution of the alpha-gal epitope on adult porcine bone tissue. Transp. Proc. 2006, 38, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Shin, J.W.; Park, S.A.; Kim, Y.K.; Park, M.S.; Mok, J.M.; Yang, W.I.; Lee, J.W. Chemical, structural properties, and osteoconductive effectiveness of bone block derived from porcine cancellous bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 68, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Guirado, J.L.; Gomez-Moreno, G.; Guardia, J.; Ortiz-Ruiz, A.; Piatelli, A.; Barone, A.; Martinez-Gonzalez, J.M.; Meseguer-Olmo, L.; Lopez-Mari, L.; Dorado, C.B. Biological response to porcine xenograft implants: An experimental study in rabbits. Implant Dent. 2012, 21, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Lu, C.T.; Feng, Z.Q.; Zhao, Q.T.; Zhou, Z.Y.; Lai, R.F. Antigen-extracted xenogeneic cancellous bone graft with recombinant human bone morphogenetic protein-2 enhances bone regeneration in repair of mandibular defect in rabbits. Kaohsiung J. Med. Sci. 2015, 31, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.K.; Tian, X.B.; Li, B.; Qiu, B.; Zhou, Z.J.; Yang, Z.; Li, Q.H. Properties of deproteinized bone for reparation of big segmental defect in long bone. Chin. J. Traumatol. 2008, 11, 152–156. [Google Scholar] [CrossRef]
- Liu, L.; Pei, F.X.; Tu, C.Q.; Zhou, Z.K.; Li, Q.H. Immunological study on the transplantation of an improved deproteinized heterogeneous bone scaffold material in tissue engineering. Chin. J. Traumatol. 2008, 11, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Arca, T.; Proffitt, J.; Genever, P. Generating 3d tissue constructs with mesenchymal stem cells and a cancellous bone graft for orthopaedic applications. Biomed. Mater. 2011, 6, 025006. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.K.; Ekser, B.; Tector, A.J. A brief history of clinical xenotransplantation. Int. J. Surg. 2015, 23, 205–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Salvatore, S.; Segreto, A.; Chiusaroli, A.; Congiu, S.; Bizzarri, F. Role of xenotransplantation in cardiac transplantation. J. Card. Surg. 2015, 30, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Manji, R.A.; Lee, W.; Cooper, D.K. Xenograft bioprosthetic heart valves: Past, present and future. Int. J. Surg. 2015, 23, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Chanchareonsook, N.; Junker, R.; Jongpaiboonkit, L.; Jansen, J.P.D. Tissue engineered mandibular bone reconstruction for continuity defects: A systematic approach to the literature. Tissue Eng. Part B Rev. 2013, 20, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Cicciu, M.; Cervino, G.; Herford, A.S.; Fama, F.; Bramanti, E.; Fiorillo, L.; Lauritano, F.; Sambataro, S.; Troiano, G.; Laino, L. Facial bone reconstruction using both marine or non-marine bone substitutes: Evaluation of current outcomes in a systematic literature review. Mar. Drugs 2018, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.C.; Mealey, B.L. Histologic comparison of healing following tooth extraction with ridge preservation using two different xenograft protocols. J. Periodontol. 2013, 84, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Herford, A.S.; Lu, M.; Akin, L.; Cicciu, M. Evaluation of a porcine matrix with and without platelet-derived growth factor for bone graft coverage in pigs. Int. J. Oral Maxillofac. Implants 2012, 27, 1351–1358. [Google Scholar] [PubMed]
- Lee, J.H.; Yi, G.S.; Lee, J.W.; Kim, D.J. Physicochemical characterization of porcine bone-derived grafting material and comparison with bovine xenografts for dental applications. J. Periodont. Implant Sci. 2017, 47, 388–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiorana, C.; Poli, P.P.; Deflorian, M.; Testori, T.; Mandelli, F.; Nagursky, H.; Vinci, R. Alveolar socket preservation with demineralised bovine bone mineral and a collagen matrix. J. Periodont. Implant Sci. 2017, 47, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Festa, V.M.; Addabbo, F.; Laino, L.; Femiano, F.; Rullo, R. Porcine-derived xenograft combined with a soft cortical membrane versus extraction alone for implant site development: A clinical study in humans. Clin. Implant Dent. Relat. Res. 2013, 15, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Ozel, B.; Findikcioglu, K.; Sezgin, B.; Guney, K.; Barut, I.; Ozmen, S. A new option for the reconstruction of orbital floor defects with heterologous cortical bone. J. Cranio Maxill. Facial Surg. 2015, 43, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Seyler, T.M.; Bracey, D.N.; Plate, J.F.; Lively, M.O.; Mannava, S.; Smith, T.L.; Saul, J.M.; Poehling, G.G.; Van Dyke, M.E.; Whitlock, P.W. The development of a xenograft-derived scaffold for tendon and ligament reconstruction using a decellularization and oxidation protocol. Arthrosc. J. Arthrosc. Relat. Surg. 2017, 33, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Stabile, K.J.; Odom, D.; Smith, T.L.; Northam, C.; Whitlock, P.W.; Smith, B.P.; Van Dyke, M.E.; Ferguson, C.M. An acellular, allograft-derived meniscus scaffold in an ovine model. Arthrosc. J. Arthrosc. Relat. Surg. 2010, 26, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, P.W.; Seyler, T.M.; Northam, C.N.; Smith, T.L.; Poehling, G.G.; Koman, L.A.; Van Dyke, M.E. Effect of cyclic strain on tensile properties of a naturally derived, decellularized tendon scaffold seeded with allogeneic tenocytes and associated messenger rna expression. J. Surg. Orthop. Adv. 2013, 22, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, P.W.; Seyler, T.M.; Parks, G.D.; Ornelles, D.A.; Smith, T.L.; Van Dyke, M.E.; Poehling, G.G. A novel process for optimizing musculoskeletal allograft tissue to improve safety, ultrastructural properties, and cell infiltration. J. Bone Jt. Surg. 2012, 94, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, P.W.; Smith, T.L.; Poehling, G.G.; Shilt, J.S.; Van Dyke, M. A naturally derived, cytocompatible, and architecturally optimized scaffold for tendon and ligament regeneration. Biomaterials 2007, 28, 4321–4329. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, J. Demineralized bone and bmps: Basic science and clinical utility. J. Oral Maxillofac. Surg. 2015, 73, S126–S131. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.M.; Nataraj, C.; Jaw, R.; Deigl, E.; Bursac, P. Demineralized bone matrix as an osteoinductive biomaterial and in vitro predictors of its biological potential. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, H.; Wu, C.H. Protein bioinformatics databases and resources. Methods Mol. Biol. 2017, 1558, 3–39. [Google Scholar] [PubMed]
- Keane, T.J.; Londono, R.; Turner, N.J.; Badylak, S.F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 2012, 33, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.T. Biology and enhancement of skeletal repair. In Skeletal Trauma: Basic Science, Management, and Reconstruction, 5th ed.; Browner, B.D., Ed.; Elsevier: Philadelphia, PA, USA, 2015; pp. 69–93. [Google Scholar]
- Arvidson, K.; Abdallah, B.M.; Applegate, L.A.; Baldini, N.; Cenni, E.; Gomez-Barrena, E.; Granchi, D.; Kassem, M.; Konttinen, Y.T.; Mustafa, K.; et al. Bone regeneration and stem cells. J. Cell. Mol. Med. 2011, 15, 718–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Long, W.G., Jr.; Einhorn, T.A.; Koval, K.; McKee, M.; Smith, W.; Sanders, R.; Watson, T. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J. Bone Surg. 2007, 89, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, A.A.; Nunley, R.M.; Mehta, S.; Sharan, A. Bone-graft substitutes in orthopaedic surgery. AAOS Now 2008, 2, 35–37. [Google Scholar]
- Bertoldi, S.; Fare, S.; Tanzi, M.C. Assessment of scaffold porosity: The new route of micro-ct. J. Appl. Biomater. Biomech. 2011, 9, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Adams, D.J.; Laurencin, C.T.; Nukavarapu, S.P. Optimally porous and biomechanically compatible scaffolds for large-area bone regeneration. Tissue Eng. Part A 2012, 18, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Wagoner Johnson, A.J. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sun, J.; Li, Y.; Zhou, H.; Cui, L.; Liu, W.; Cao, Y. Evaluation of partially demineralized osteoporotic cancellous bone matrix combined with human bone marrow stromal cells for tissue engineering: An in vitro and in vivo study. Calcif. Tissue Int. 2008, 83, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Campos, I.; Marolt, D.; Petridis, P.; Bhumiratana, S.; Schmidt, D.; Vunjak-Novakovic, G. Bone scaffold architecture modulates the development of mineralized bone matrix by human embryonic stem cells. Biomaterials 2012, 33, 8329–8342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pneumaticos, S.G.; Triantafyllopoulos, G.K.; Basdra, E.K.; Papavassiliou, A.G. Segmental bone defects: From cellular and molecular pathways to the development of novel biological treatments. J. Cell. Mol. Med. 2010, 14, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Li, Z.; Luo, F.; Xie, Z.; Wu, X.; Xing, J.; Dong, S.; Xu, J. A composite demineralized bone matrix—Self assembling peptide scaffold for enhancing cell and growth factor activity in bone marrow. Biomaterials 2014, 35, 5689–5699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mao, X.; Du, Z.; Jiang, W.; Han, X.; Zhao, D.; Han, D.; Li, Q. Three dimensional printed macroporous polylactic acid/hydroxyapatite composite scaffolds for promoting bone formation in a critical-size rat calvarial defect model. Sci. Technol. Adv. Mater. 2016, 17, 136–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwiedrzik, J.J.; Kaudela, K.H.; Burner, U.; Zysset, P.K. Fabric-mechanical property relationships of trabecular bone allografts are altered by supercritical co(2) treatment and gamma sterilization. Bone 2011, 48, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, T.; Laib, A.; Muller, R.; Dequeker, J.; Ruegsegger, P. Direct three-dimensional morphometric analysis of human cancellous bone: Microstructural data from spine, femur, iliac crest, and calcaneus. J. Bone Miner. Res. 1999, 14, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, S.; Hart, R.A. The need for structural allograft biomechanical guidelines. J. Am. Acad. Orthop. Surg. 2015, 23, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.S.; Barrows, T.H.; Cartmell, S.H.; Guldberg, R.E. Microarchitectural and mechanical characterization of oriented porous polymer scaffolds. Biomaterials 2003, 24, 481–489. [Google Scholar] [CrossRef]
- Novitskaya, E.; Chen, P.Y.; Lee, S.; Castro-Cesena, A.; Hirata, G.; Lubarda, V.A.; McKittrick, J. Anisotropy in the compressive mechanical properties of bovine cortical bone and the mineral and protein constituents. Acta Biomater. 2011, 7, 3170–3177. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.J.; Ramsey, D.S.; Dux, S.J.; Chu, E.H.; Rimnac, C.M. Irradiation does not modify mechanical properties of cancellous bone under compression. Clin. Orthop. Relat. Res. 2012, 470, 2488–2495. [Google Scholar] [CrossRef] [PubMed]
- Haimi, S.; Vienonen, A.; Hirn, M.; Pelto, M.; Virtanen, V.; Suuronen, R. The effect of chemical cleansing procedures combined with peracetic acid-ethanol sterilization on biomechanical properties of cortical bone. Biologicals 2008, 36, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Cornu, O.; Banse, X.; Docquier, P.L.; Luyckx, S.; Delloye, C. Effect of freeze-drying and gamma irradiation on the mechanical properties of human cancellous bone. J. Orthop. Res. 2000, 18, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Kemper, N.; Davison, N.; Fitzpatrick, D.; Marshall, R.; Lin, A.; Mundy, K.; Cobb, R.R. Characterization of the mechanical properties of bovine cortical bone treated with a novel tissue sterilization process. Cell Tissue Bank. 2011, 12, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Liu, Y.; Lim, J.; Teoh, S.H. Review: Development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol. Adv. 2013, 31, 688–705. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Billstrom, G.H.; Blom, A.W.; Larsson, S.; Beswick, A.D. Application of scaffolds for bone regeneration strategies: Current trends and future directions. Injury 2013, 44 (Suppl. 1), S28–S33. [Google Scholar] [CrossRef]
- Shah, N.; Morsi, Y.; Manasseh, R. From mechanical stimulation to biological pathways in the regulation of stem cell fate. Cell Biochem. Funct. 2014, 32, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Watari, S.; Hayashi, K.; Wood, J.A.; Russell, P.; Nealey, P.F.; Murphy, C.J.; Genetos, D.C. Modulation of osteogenic differentiation in hmscs cells by submicron topographically-patterned ridges and grooves. Biomaterials 2012, 33, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Behnam, K.; Murray, S.S.; Brochmann, E.J. Bmp stimulation of alkaline phosphatase activity in pluripotent mouse C2C12 cells is inhibited by dermatopontin, one of the most abundant low molecular weight proteins in demineralized bone matrix. Connect. Tissue Res. 2006, 47, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Behnam, K.; Murray, S.S.; Whitelegge, J.P.; Brochmann, E.J. Identification of the molecular chaperone alpha b-crystallin in demineralized bone powder and osteoblast-like cells. J. Orthop. Res. 2002, 20, 1190–1196. [Google Scholar] [CrossRef]
- Rodriguez, R.U.; Kemper, N.; Breathwaite, E.; Dutta, S.M.; Hsu, E.L.; Hsu, W.K.; Francis, M.P. Demineralized bone matrix fibers formable as general and custom 3d printed mold-based implants for promoting bone regeneration. Biofabrication 2016, 8, 035007. [Google Scholar] [CrossRef] [PubMed]
- Cicciu, M.; Herford, A.S.; Cicciu, D.; Tandon, R.; Maiorana, C. Recombinant human bone morphogenetic protein-2 promote and stabilize hard and soft tissue healing for large mandibular new bone reconstruction defects. J. Craniofac. Surg. 2014, 25, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Herford, A.S.; Cicciu, M.; Eftimie, L.F.; Miller, M.; Signorino, F.; Fama, F.; Cervino, G.; Lo Giudice, G.L.; Bramanti, E.; Lauritano, F.; et al. Rhbmp-2 applied as support of distraction osteogenesis: A split-mouth histological study over nonhuman primates mandibles. Int. J. Clin. Exp. Med. 2016, 9, 17187–17194. [Google Scholar]
- Monje, A.; Monje, F.; Hernandez-Alfaro, F.; Gonzalez-Garcia, R.; Suarez-Lopez del Amo, F.; Galindo-Moreno, P.; Montanero-Fernandez, J.; Wang, H.L. Horizontal bone augmentation using autogenous block grafts and particulate xenograft in the severe atrophic maxillary anterior ridges: A cone-beam computerized tomography case series. J. Oral Implantol. 2015, 41, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Yon, J.; Lee, J.S.; Lim, H.C.; Kim, M.S.; Hong, J.Y.; Choi, S.H.; Jung, U.W. Pre-clinical evaluation of the osteogenic potential of bone morphogenetic protein-2 loaded onto a particulate porcine bone biomaterial. J. Clin. Periodontol. 2015, 42, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, P.W.; Van Dyke, M.E.; Christ, G.J. Structurally Modified Acellular Tissue Engineering Scaffolds and Methods of Production. U.S. Patent No. 8,221,777 B2, 17 July 2012. [Google Scholar]
- Jaecques, S.V.; Van Oosterwyck, H.; Muraru, L.; Van Cleynenbreugel, T.; De Smet, E.; Wevers, M.; Naert, I.; Vander Sloten, J. Individualised, micro ct-based finite element modelling as a tool for biomechanical analysis related to tissue engineering of bone. Biomaterials 2004, 25, 1683–1696. [Google Scholar] [CrossRef]
- Ho, S.T.; Hutmacher, D.W. A comparison of micro ct with other techniques used in the characterization of scaffolds. Biomaterials 2006, 27, 1362–1376. [Google Scholar] [CrossRef] [PubMed]
- Mikhael, M.M.; Huddleston, P.M.; Zobitz, M.E.; Chen, Q.; Zhao, K.D.; An, K.N. Mechanical strength of bone allografts subjected to chemical sterilization and other terminal processing methods. J. Biomech. 2008, 41, 2816–2820. [Google Scholar] [CrossRef] [PubMed]
- Mroz, T.E.; Lin, E.L.; Summit, M.C.; Bianchi, J.R.; Keesling, J.E., Jr.; Roberts, M.; Vangsness, C.T., Jr.; Wang, J.C. Biomechanical analysis of allograft bone treated with a novel tissue sterilization process. Spine J. 2006, 6, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Ishihama, Y.; Oda, Y.; Tabata, T.; Sato, T.; Nagasu, T.; Rappsilber, J.; Mann, M. Exponentially modified protein abundance index (empai) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell. Proteom. 2005, 4, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Thibault, R.A.; Mikos, A.G.; Kasper, F.K. Protein and mineral composition of osteogenic extracellular matrix constructs generated with a flow perfusion bioreactor. Biomacromolecules 2011, 12, 4204–4212. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Donor Bone | Scaffold | |
|---|---|---|---|
| Density | 1366 ± 20 mg/mL | 570 ± 9 mg/mL | p < 0.01 |
| Porosity | 79.5 ± 9.1% | 69.1 ± 11.1% | p = 0.2 |
| Anisotropy | 1.88 ± 0.1 | 1.65 ± 0.1 | p = 0.1 |
| Mean Pore Size | 458.5 ± 66.3 µm | 474.2 ± 76.2 µm | p = 0.8 |
| Strut Thickness | 142.8 ± 27.8 µm | 121.7 ± 21.9 µm | p = 0.3 |
| Donor Bone | Scaffold | ||
|---|---|---|---|
| FEA Modeling | |||
| Yield Stress (von Mises) | 11,372 ± 286 MPa | 10,922 ± 327 MPa | p = 0.39 |
| Stiffness | 31,921± 8250 N/mm | 18,840 ± 6603 N/mm | p = 0.26 |
| Failure Load | 148.0 ± 35.7 MPa | 89.5 ± 29.5 MPa | p = 0.25 |
| Mechanical Testing | |||
| Young’s Modulus | 236.6 ± 11.8 MPa | 114.2 ± 17.8 MPa | p < 0.01 |
| Stiffness | 1544.0 ± 76.2 N/mm | 727.6 ± 120.1 N/mm | p < 0.01 |
| Failure Load | 14.5 ± 1.8 MPa | 13.6 ± 1.8 MPa | p = 0.72 |
| Strain at Failure | 0.088 ± 0.006 | 0.230 ± 0.014 | p < 0.01 |
| Protein Detected | UniProtKB # | Porcine Scaffold Samples | Human DBM Samples | Protein Function |
|---|---|---|---|---|
| Chondroadherin | F1RT93 O15335 | 3/3 | 3/3 | Promotes the attachment of chondrocytes, fibroblasts, and osteoblasts. JAK-STAT cascade signaling |
| Collagen alpha-1(I) chain | P02452 | 3/3 | 3/3 | Protease binding, metal ion binding, bone trabeculae formation, enchondral ossification, cell response to TGF β, collagen fibril organization, cell response to mechanical stimuli, and osteoblast differentiation |
| Collagen alpha-2(I) chain | A0A1S7J1Y9 P08123 | 3/3 | 3/3 | Extracellular matrix structural constituent, SMAD signaling, collagen fibril organization, cytokine signaling, and TGF β receptor signaling |
| Pigment epithelium-derived factor | Q0PM28 P36955 | 3/3 | 3/3 | Neurotrophic protein, inhibitor of angiogenesis, cell proliferation |
| Serum albumin | P08835 P02768 | 3/3 | 3/3 | Main plasma protein |
| Alpha-2-HS-glycoprotein | P29700 P02765 | 3/3 | 2/3 | Endopeptidase inhibitor; negative regulation of biomineral tissue development; negative regulation of bone mineralization, positive regulation of phagocytosis |
| Lumican; fibromodulin | Q9TTB4 P51884 | 3/3 | 2/3 | Primary role in collagen fibrillogenesis, collagen binding, response to growth factor |
| Biglycan | Q9GKQ6 P21810 | 2/3 | 3/3 | Involved in collagen fiber assembly, protein kinase inhibitor, negative regulation of JAK-STAT cascade, ECM structure, blood vessel remodeling, and cytokine signaling |
| Annexin A5 | P08758 | 3/3 | 0/3 | Blood coagulation |
| Hemoglobin subunit beta | P02067 P68871 | 2/3 | 0/3 | Highly abundant blood protein, oxygen transport |
| Alpha-1-antiproteinase | P50447 P01009 | 2/3 | 0/3 | Serine protease inhibitor; found in high levels in blood |
| Vitronectin | P48819 P04004 | 0/3 | 3/3 | Cell adhesion and spreading factor in serum and tissues, cell proliferation, wound healing, and cell migration |
| Prothrombin | F1SIB1 P00734 | 0/3 | 2/3 | Blood coagulation, converts fibrinogen to fibrin, activates coagulation factors |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bracey, D.N.; Seyler, T.M.; Jinnah, A.H.; Lively, M.O.; Willey, J.S.; Smith, T.L.; Van Dyke, M.E.; Whitlock, P.W. A Decellularized Porcine Xenograft-Derived Bone Scaffold for Clinical Use as a Bone Graft Substitute: A Critical Evaluation of Processing and Structure. J. Funct. Biomater. 2018, 9, 45. https://doi.org/10.3390/jfb9030045
Bracey DN, Seyler TM, Jinnah AH, Lively MO, Willey JS, Smith TL, Van Dyke ME, Whitlock PW. A Decellularized Porcine Xenograft-Derived Bone Scaffold for Clinical Use as a Bone Graft Substitute: A Critical Evaluation of Processing and Structure. Journal of Functional Biomaterials. 2018; 9(3):45. https://doi.org/10.3390/jfb9030045
Chicago/Turabian StyleBracey, Daniel N., Thorsten M. Seyler, Alexander H. Jinnah, Mark O. Lively, Jeffrey S. Willey, Thomas L. Smith, Mark E. Van Dyke, and Patrick W. Whitlock. 2018. "A Decellularized Porcine Xenograft-Derived Bone Scaffold for Clinical Use as a Bone Graft Substitute: A Critical Evaluation of Processing and Structure" Journal of Functional Biomaterials 9, no. 3: 45. https://doi.org/10.3390/jfb9030045
APA StyleBracey, D. N., Seyler, T. M., Jinnah, A. H., Lively, M. O., Willey, J. S., Smith, T. L., Van Dyke, M. E., & Whitlock, P. W. (2018). A Decellularized Porcine Xenograft-Derived Bone Scaffold for Clinical Use as a Bone Graft Substitute: A Critical Evaluation of Processing and Structure. Journal of Functional Biomaterials, 9(3), 45. https://doi.org/10.3390/jfb9030045