Multifunctional PLA Blends Containing Chitosan Mediated Silver Nanoparticles: Thermal, Mechanical, Antibacterial, and Degradation Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
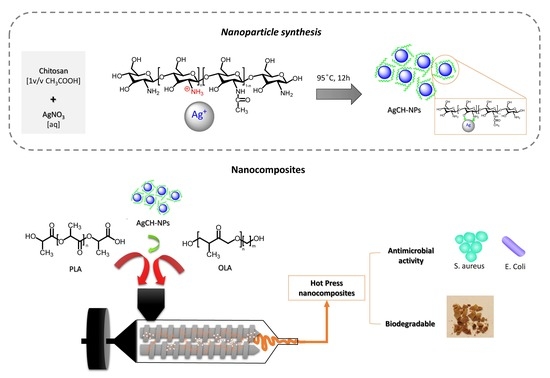
2.2. Synthesis of Based Chitosan Silver Nanoparticles
2.3. Preparation of Oligomeric Lactic Acid Plasticized Poly(Lactic Acid) Nanocomposites Containing AgCH-NPs (PLA/OLA AgCH-NPs Nanocomposites)
2.4. Characterization Techniques
3. Results
3.1. Microstructure and Morphology of the PLA/OLA AgCH-NPs Nanocomposites:
3.2. Thermal Analysis
3.3. Mechanical Properties
3.4. Antibacterial Activity
3.5. Disintegration under Composting Conditions of PLA/OLA AgCH-NPs Nanocomposites:
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, H.S.; Yoon, J.S.; Kim, M.N. Dependence of biodegradability of plastics in compost on the shape of specimens. Polym. Degrad. Stab. 2005, 87, 131–135. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Garreau, H.; Vert, M. Selective enzymatic degradations of poly(L-lactide) and poly(∈-caprolactone) blend films. Biomacromolecules 2000, 1, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Mattioli, S.; Peltzer, M.; Fortunati, E.; Armentano, I.; Jiménez, A.; Kenny, J.M. Structure, gas-barrier properties and overall migration of poly(lactic acid) films coated with hydrogenated amorphous carbon layers. Carbon N. Y. 2013, 63, 274–282. [Google Scholar] [CrossRef]
- Rhim, J.W.; Hong, S.I.; Ha, C.S. Tensile, water vapor barrier and antimicrobial properties of PLA/nanoclay composite films. LWT—Food Sci. Technol. 2009, 42, 612–617. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Blends of aliphatic polyesters. II. Hydrolysis of solution-cast blends from poly(L-lactide) and poly(Ε-caprolactone) in phosphate-buffered solution. J. Appl. Polym. Sci. 1998, 67, 405–415. [Google Scholar] [CrossRef]
- Fukushima, K.; Abbate, C.; Tabuani, D.; Gennari, M.; Camino, G. Biodegradation of poly(lactic acid) and its nanocomposites. Polym. Degrad. Stab. 2009, 94, 1646–1655. [Google Scholar] [CrossRef]
- Petersen, K.; Nielsen, P.V.; Olsen, M.B. Physical and mechanical properties of biobased materials—Starch, polylactate and polyhydroxybutyrate. Starch/Staerke 2001, 53, 356–361. [Google Scholar] [CrossRef]
- Hiljanen-Vainio, M.; Varpomaa, P.; Seppälä, J.; Törmälä, P. Modification of poly(L-lactides) by blending: Mechanical and hydrolytic behavior. Macromol. Chem. Phys. 1996, 197, 1503–1523. [Google Scholar] [CrossRef]
- Davoodi, S.; Oliaei, E.; Davachi, S.M.; Hejazi, I.; Seyfi, J.; Heidari, B.S.; Ebrahimi, H. Preparation and characterization of interface-modified PLA/starch/PCL ternary blends using PLLA/triclosan antibacterial nanoparticles for medical applications. RSC Adv. 2016, 6, 39870–39882. [Google Scholar] [CrossRef]
- Khakbaz, M.; Hejazi, I.; Seyfi, J.; Davachi, S.M.; Jafari, S.H.; Khonakdar, H.A. Study on the effects of non-solvent and nanoparticle concentrations on surface properties of water-repellent biocompatible l-lactide/glycolide/trimethylene carbonate terpolymers. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 502, 168–175. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Sain, M. Mechanical properties of biodegradable composites from poly lactic acid (PLA) and microcrystalline cellulose (MCC). J. Appl. Polym. Sci. 2005, 97, 2014–2025. [Google Scholar] [CrossRef]
- Davachi, S.M.; Shiroud Heidari, B.; Hejazi, I.; Seyfi, J.; Oliaei, E.; Farzaneh, A.; Rashedi, H. Interface modified polylactic acid/starch/poly ε-caprolactone antibacterial nanocomposite blends for medical applications. Carbohydr. Polym. 2017, 155, 336–344. [Google Scholar] [CrossRef]
- Cierpiszewski, R.; Korzeniowski, A.; Dobrucka, R. Intelligent food packaging—Research and development. LogForum 2015, 11, 7–14. [Google Scholar]
- Chaudhry, Q.; Scotter, M.; Blackburn, J.; Ross, B.; Boxall, A.; Castle, L.; Aitken, R.; Watkins, R. Applications and implications of nanotechnologies for the food sector. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Mahalik, N. Advances in Packaging Methods, Processes and Systems. Challenges 2014, 5, 374–389. [Google Scholar] [CrossRef] [Green Version]
- Koedrith, P.; Thasiphu, T.; Tuitemwong, K.; Boonprasert, R.; Tuitemwong, P. Recent advances in potential nanoparticles and nanotechnology for sensing food-bome pathogens and their toxins in foods and crops: Current technologies and limitations. Sensors Mater. 2014, 26, 711–736. [Google Scholar]
- Amaya-González, S.; de-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Aptamer-based analysis: A promising alternative for food safety control. Sensors 2013, 13, 16292–16311. [Google Scholar] [CrossRef] [Green Version]
- Vermeiren, L.; Devlieghere, F.; Van Beest, M.; De Kruijf, N.; Debevere, J. Developments in the active packaging of foods. Trends Food Sci. Technol. 1999, 10, 77–86. [Google Scholar] [CrossRef]
- Oloffs, A.; Grosse-Siestrup, C.; Bisson, S.; Rinck, M.; Rudolph, R.; Gross, U. Biocompatibility of silver-coated polyurethane catheters and silvercoated Dacron® material. Biomaterials 1994, 15, 753–758. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. The roadmap of antimicrobial polymeric materials in macromolecular nanotechnology. Eur. Polym. J. 2015, 65, 46–62. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Paino, M.; Muñoz-Bonilla, A.; Fernández-García, M. Antimicrobial polymers in the nano-world. Nanomaterials 2017, 7, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.P.; Fernández-García, M. Bio-based polymers with antimicrobial properties towards sustainable development. Materials 2019, 12, 641. [Google Scholar] [CrossRef] [Green Version]
- Quintavalla, S.; Vicini, L. Antimicrobial food packaging in meat industry. Meat Sci. 2002, 62, 373–380. [Google Scholar] [CrossRef]
- Chowdhury, N.R.; Cowin, A.J.; Zilm, P.; Vasilev, K. “Chocolate” gold nanoparticles—one pot synthesis and biocompatibility. Nanomaterials 2018, 8, 496. [Google Scholar] [CrossRef] [Green Version]
- Slepička, P.; Slepičková Kasálková, N.; Pinkner, A.; Sajdl, P.; Kolská, Z.; Švorčík, V. Plasma induced cytocompatibility of stabilized poly-L-lactic acid doped with graphene nanoplatelets. React. Funct. Polym. 2018, 131, 266–275. [Google Scholar] [CrossRef]
- Murugadoss, A.; Chattopadhyay, A. A “green” chitosan-silver nanoparticle composite as a heterogeneous as well as micro-heterogeneous catalyst. Nanotechnology 2008, 19. [Google Scholar] [CrossRef]
- Sanpui, P.; Murugadoss, A.; Prasad, P.V.D.; Ghosh, S.S.; Chattopadhyay, A. The antibacterial properties of a novel chitosan-Ag-nanoparticle composite. Int. J. Food Microbiol. 2008, 124, 142–146. [Google Scholar] [CrossRef]
- Abdel-Mohsen, A.M.; Abdel-Rahman, R.M.; Fouda, M.M.G.; Vojtova, L.; Uhrova, L.; Hassan, A.F.; Al-Deyab, S.S.; El-Shamy, I.E.; Jancar, J. Preparation, characterization and cytotoxicity of schizophyllan/silver nanoparticle composite. Carbohydr. Polym. 2014, 102, 238–245. [Google Scholar] [CrossRef]
- Abdel-Mohsen, A.M.; Hrdina, R.; Burgert, L.; Krylová, G.; Abdel-Rahman, R.M.; Krejčová, A.; Steinhart, M.; Beneš, L. Green synthesis of hyaluronan fibers with silver nanoparticles. Carbohydr. Polym. 2012, 89, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mohsen, A.M.; Aly, A.S.; Hrdina, R.; El-Aref, A.T. A novel method for the preparation of silver/chitosan-O-methoxy polyethylene glycol core shell nanoparticles. J. Polym. Environ. 2012, 20, 459–468. [Google Scholar] [CrossRef]
- Kalaivani, R.; Maruthupandy, M.; Muneeswaran, T.; Hameedha Beevi, A.; Anand, M.; Ramakritinan, C.M.; Kumaraguru, A.K. Synthesis of chitosan mediated silver nanoparticles (Ag NPs) for potential antimicrobial applications. Front. Lab. Med. 2018, 2, 30–35. [Google Scholar] [CrossRef]
- Venkatesham, M.; Ayodhya, D.; Madhusudhan, A.; Veera Babu, N.; Veerabhadram, G. A novel green one-step synthesis of silver nanoparticles using chitosan: Catalytic activity and antimicrobial studies. Appl. Nanosci. 2014, 4, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Wei, D.; Sun, W.; Qian, W.; Ye, Y.; Ma, X. The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr. Res. 2009, 344, 2375–2382. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid-Zeitschrift Zeitschrift Für Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- ASTM International, ASTM E2149: Standard Test Method for Determining the Antimicrobial Activity of Antimicrobial Agents Under Dynamic Contact Conditions. Available online: https://www.astm.org/Standards/E2149.html (accessed on 18 December 2019).
- International Standard, ISO20200:2015: Determination of the degree of disintegration of plastic materials under simulated composting conditions in a laboratory-scale test: International Standard. Available online: https://www.iso.org/standard/63367.html (accessed on 18 December 2019).
- Chu, Z.; Zhao, T.; Li, L.; Fan, J.; Qin, Y. Characterization of antimicrobial poly (lactic acid)/nano-composite films with silver and zinc oxide nanoparticles. Materials 2017, 10, 659. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, S.; Asakura, T. Helix distortion and crystal structure of the α-form of poly(L-lactide). Macromolecules 2003, 36, 8385–8390. [Google Scholar] [CrossRef]
- Bautista-Del-Ángel, J.E.; Morales-Cepeda, A.B.; Lozano-Ramírez, T.; Sanchez, S.; Karami, S.; Lafleur, P. Enhancement of crystallinity and toughness of poly (L-lactic acid) influenced by Ag nanoparticles processed by twin screw extruder. Polym. Compos. 2018, 39, 2368–2376. [Google Scholar] [CrossRef]
- Pan, P.; Kai, W.; Zhu, B.; Dong, T.; Inoue, Y. Polymorphous crystallization and multiple melting behavior of poly(L-lactide): Molecular weight dependence. Macromolecules 2007, 40, 6898–6905. [Google Scholar] [CrossRef]
- Miyata, T.; Masuko, T. Morphology of poly(L-lactide) solution-grown crystals. Polymer (Guildf) 1997, 38, 4003–4009. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Li, J.; Zou, Q.; Zuo, Y.; Tian, W.; Li, Y. Physicochemical and biological properties of nano-hydroxyapatite-reinforced aliphatic polyurethanes membranes. J. Biomater. Sci. Polym. Ed. 2010, 21, 1619–1636. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Baena, I.; Arrieta, M.P.M.P.; Sonseca, A.; Torre, L.; López, D.; Giménez, E.; Kenny, J.M.J.M.; Peponi, L. Biodegradable nanocomposites based on poly(ester-urethane) and nanosized hydroxyapatite: Plastificant and reinforcement effects. Polym. Degrad. Stab. 2015, 121, 171–179. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Chow, L.C.; Yang, M.; Mitchell, J.W. Effects of Inorganic Fillers on the Thermal and Mechanical Properties of Poly(lactic acid). Int. J. Polym. Sci. 2014, 2014, 827028. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Girard, A.; Garreau, H.; Vert, M. Enzymatic degradation of polylactide stereocopolymers with predominant D-lactyl contents. Polym. Degrad. Stab. 2000, 71, 61–67. [Google Scholar] [CrossRef]
- Li, S.; McCarthy, S. Further investigations on the hydrolytic degradation of poly (DL-lactide). Biomaterials 1999, 20, 35–44. [Google Scholar] [CrossRef]
- Khabbaz, F.; Karlsson, S.; Albertsson, A.C. Py-GC/MS an effective technique to characterizing of degradation mechanism of poly (L-lactide) in the different environment. J. Appl. Polym. Sci. 2000, 78, 2369–2378. [Google Scholar] [CrossRef]












| Sample | PLA (wt%) | OLA (wt%) | AgCH-NPs (wt%) |
|---|---|---|---|
| PLA/OLA | 80 | 20 | 0 |
| PLA/OLA-AgCH-0.5% | 79.6 | 20 | 0.4 |
| PLA/OLA-AgCH-1% | 79.2 | 20 | 0.8 |
| PLA/OLA-AgCH-3% | 77.6 | 20 | 2.4 |
| PLA/OLA-AgCH-5% | 76 | 20 | 4 |
| 2θ (Angle) | Distance between Planes | |||||
|---|---|---|---|---|---|---|
| Sample | (010) | (200)/(110) | (203) | d(A) | d(A) | d(A) |
| PLA/OLA AgCH1% | -- | 16.7 | -- | -- | 5.30 | -- |
| PLA/OLA AgCH3% | 14.8 | 16.7 | 19.1 | 5.98 | 5.30 | 4.64 |
| PLA/OLA AgCH5% | 14.9 | 16.8 | 19.2 | 5.94 | 5.27 | 4.62 |
| Sample | Temperature at Maximum Weight Loss Rates (°C) | Temperature at Different Weight Losses (°C) | ||||
|---|---|---|---|---|---|---|
| Tmax1 | Tmax2 | T5 | T30 | T50 | T70 | |
| PLA/OLA | 306 | 331 | 227 | 284 | 304 | 321 |
| PLA/OLA AgCH0.5% | 312 | 341 | 224 | 285 | 304 | 322 |
| PLA/OLA AgCH1% | 273 | 296 | 211 | 258 | 270 | 279 |
| PLA/OLA AgCH3% | 275 | -- | 203 | 253 | 266 | 276 |
| PLA/OLA AgCH5% | 303 | -- | 210 | 268 | 284 | 299 |
| Sample | Tg | Tcc | ΔHcc | Tm | ΔHm | ΔHTotal | Xc-DSC (%) | Xc-XRD (%) |
|---|---|---|---|---|---|---|---|---|
| PLA/OLA | 32 | 88 | 25 | 143 | 27 | 2 | 2.8 | -- |
| PLA/OLA AgCH0.5% | 25 | 83 | 27 | 142 | 27 | 0 | 0.0 | -- |
| PLA/OLA AgCH1% | 24 | 76 | 23 | 142 | 29 | 6 | 9.2 | 3.3 |
| PLA/OLA AgCH3% | 50 | 66 | 2 | 142 | 30 | 28 | 38.0 | 26.2 |
| PLA/OLA AgCH5% | 53 | 68 | 1 | 141 | 27 | 26 | 37.5 | 21.9 |
| Sample | E (MPa) | ε (%) | σmax (MPa) | Toughness (MJ/m3) |
|---|---|---|---|---|
| PLA/OLA | 783 ± 102 | 108 ± 6 | 23 ± 2 | 1.8 ± 0.1 |
| PLA/OLA AgCH0.5% | 256 ± 29 | 372 ± 26 | 23 ± 2 | 5.2 ± 0.7 |
| PLA/OLA AgCH1% | 88 ± 13 | 368 ± 32 | 16 ± 1 | 3.1 ± 0.6 |
| PLA/OLA AgCH3% | 123 ± 36 | 369 ± 50 | 16 ± 2 | 3.3 ± 0.5 |
| PLA/OLA AgCH5% | 132 ± 29 | 338 ± 51 | 14 ± 3 | 3.1 ± 0.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonseca, A.; Madani, S.; Rodríguez, G.; Hevilla, V.; Echeverría, C.; Fernández-García, M.; Muñoz-Bonilla, A.; Charef, N.; López, D. Multifunctional PLA Blends Containing Chitosan Mediated Silver Nanoparticles: Thermal, Mechanical, Antibacterial, and Degradation Properties. Nanomaterials 2020, 10, 22. https://doi.org/10.3390/nano10010022
Sonseca A, Madani S, Rodríguez G, Hevilla V, Echeverría C, Fernández-García M, Muñoz-Bonilla A, Charef N, López D. Multifunctional PLA Blends Containing Chitosan Mediated Silver Nanoparticles: Thermal, Mechanical, Antibacterial, and Degradation Properties. Nanomaterials. 2020; 10(1):22. https://doi.org/10.3390/nano10010022
Chicago/Turabian StyleSonseca, Agueda, Salim Madani, Gema Rodríguez, Víctor Hevilla, Coro Echeverría, Marta Fernández-García, Alexandra Muñoz-Bonilla, Noureddine Charef, and Daniel López. 2020. "Multifunctional PLA Blends Containing Chitosan Mediated Silver Nanoparticles: Thermal, Mechanical, Antibacterial, and Degradation Properties" Nanomaterials 10, no. 1: 22. https://doi.org/10.3390/nano10010022
APA StyleSonseca, A., Madani, S., Rodríguez, G., Hevilla, V., Echeverría, C., Fernández-García, M., Muñoz-Bonilla, A., Charef, N., & López, D. (2020). Multifunctional PLA Blends Containing Chitosan Mediated Silver Nanoparticles: Thermal, Mechanical, Antibacterial, and Degradation Properties. Nanomaterials, 10(1), 22. https://doi.org/10.3390/nano10010022