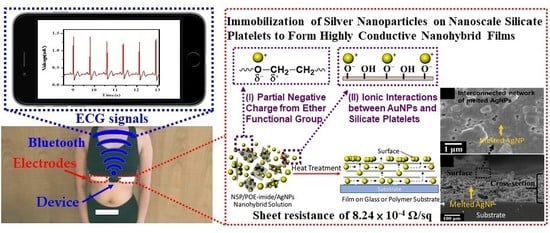
Facile Fabrication of Flexible Electrodes and Immobilization of Silver Nanoparticles on Nanoscale Silicate Platelets to Form Highly Conductive Nanohybrid Films for Wearable Electronic Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Direct Exfoliation of Layered Na+-MMT Clay into Nanoscale Silicate Platelets in Water Suspension
2.3. Synthesis of the Poly(oxyethelene)-Segmented Amide–Imide
2.4. Preparation of NSP/POE-Imide/AgNP Nanohybrid Suspensions
2.5. Preparation of NSP/POE-Imide/AgNP Nanohybrid Films
2.6. Instrumental Characterization
3. Results and Discussion
3.1. Synthesis of NSP/POE-Imide/AgNP Nanohybrid Suspensions
3.2. Facile Fabrication of the Highly Conductive NSP/POE-Imide/AgNPs Nanohybrid Films
3.3. Observation of Interconnected Network of Melted AgNPs via FE-SEM
3.4. Measurements of ECG Signals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- An, B.W.; Shin, J.H.; Kim, S.Y.; Kim, J.; Ji, S.; Park, J.; Lee, Y.; Jang, J.; Park, Y.G.; Cho, E.; et al. Smart sensor systems for wearable electronic devices. Polymers 2017, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Pandian, P.S.; Mohanavelu, K.; Safeer, K.P.; Kotresh, T.M.; Shakunthala, D.T.; Gopal, P.; Padaki, V.C. Smart vest: Wearable multi-parameter remote physiological monitoring system. Med. Eng. Phys. 2008, 30, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Celik, N.; Manivannan, N.; Strudwick, A.; Balanchendran, W. Graphene-enabled electrodes for electrocardiogram monitoring. Nanomaterials 2016, 6, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcsharry, P.E.; Clifford, G.D.; Tarassenko, L.; Smith, L.A. A dynamical model for generating synthetic electrocardiogram signals. IEEE Trans. Biomed. Eng. 2003, 5, 289–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Shin, S.; Lee, S.; Seo, J.; Son, S.; Cho, H.J.; Algadi, H.; Al-Sayari, S.; Kim, D.E.; Lee, T. Ag nanowire reinforced highly stretchable conductive fibers for wearable electronics. Adv. Funct. Mater. 2015, 25, 3114–3121. [Google Scholar] [CrossRef]
- Dong, S.; Han, B.; Ou, J.; Li, Z.; Han, L.; Yu, X. Electrically conductive behaviors and mechanisms of short-cut super-fine stainless wire reinforced reactive powder concrete. Cem. Concr. Compos. 2016, 720, 48–65. [Google Scholar] [CrossRef] [Green Version]
- Wei, D.; Cotton, D.; Ryhanen, T. All-solid-state textile batteries made from nano-emulsion conducting polymer inks for wearable electronics. Nanomaterials 2012, 2, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Jost, K.; Stenger, D.; Perez, C.R.; Mcdonough, J.K.; Lian, K.; Gogotsi, Y.; Dion, G. Knitted and screen printed carbon-fiber supercapacitors for applications in wearable electronics. Energy Environ. Sci. 2013, 6, 2698–2705. [Google Scholar] [CrossRef]
- Su, M.; Li, F.; Chen, S.; Huang, Z.; Qin, M.; Li, W.; Zhang, X.; Song, Y. Nanoparticle based curve arrays for multirecognition flexible electronics. Adv. Mater. 2015, 28, 1369–1374. [Google Scholar] [CrossRef]
- Faupel, F.; Zaporojtchenko, V.; Strunskus, T.; Elbahri, M. Metal-polymer nanocomposites for functional applications. Adv. Eng. Mater. 2010, 12, 1177–1190. [Google Scholar] [CrossRef]
- Vojtech, L.; Bortel, R.; Neruda, M.; Kozak, M. Wearable textile electrodes for ECG measurement. Adv. Electr. Electron. Eng. 2013, 11, 410–414. [Google Scholar] [CrossRef]
- Kumar, P.; Gusain, M.; Nagarajan, R. Synthesis of Cu1.8S and CuS from copper-thiourea containing precursors; anionic (ClP−, NO3−, SO4P2−P) influence on the product stoichiometry. Inorg. Chem. 2011, 50, 3065–3070. [Google Scholar] [CrossRef] [PubMed]
- Magdassi, S.; Grouchko, M.; Kamyshny, A. Copper nanoparticles for printed electronics: Routes towards achieving oxidation stability. Materials 2010, 3, 4626–4638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tursunkulov, O.; Allabergenov, B.; Abidov, A.; Jeong, S.W.; Kim, S. Synthesis, characterization and functionalization of the coated iron oxide nanostructures. J. Korean Powd. Met. Inst. 2013, 20, 180. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; He, C.L.; Peng, Y.; Wang, J.; Long, X.; Li, P.; Chan, A.S.C. Magnetic and conductive Fe3O4− polyaniline nanoparticles with core–shell structure. Synth. Met. 2003, 139, 295–301. [Google Scholar] [CrossRef]
- Chiu, C.W.; Ou, G.B.; Tsai, Y.H.; Lin, J.J. Immobilization of silver nanoparticles on exfoliated mica nanosheets to form highly conductive nanohybrid films. Nanotechnology 2015, 26, 465702. [Google Scholar] [CrossRef]
- Wang, S.; He, M.; Weng, B.; Gan, L.; Zhao, Y.; Xie, Y. Stretchable and wearable triboelectric nanogenerator based on kinesio tape for self-powered human motion sensing. Nanomaterials 2018, 8, 657. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.W.; Lin, C.A.; Hong, P.D. Melt-Spinning and thermal stability behaviour of TiO2 nanoparticle/polypropylene nanocomposite fibers. J. Polym. Res. 2011, 18, 367–372. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Kholoud, M.M.; Abou, E.N.; Ala, E.; Abdulrhman, A.W. Synthesis and applications of silver nanoparticles. Arabian J. Chem. 2010, 3, 135–140. [Google Scholar]
- Chiu, C.W.; Ou, G.B. Facile preparation of highly electrically conductive films of silver nanoparticles finely dispersed in polyisobutylene-b-poly(oxyethylene)-b-polyisobutylene triblock copolymers and graphene oxide hybrid surfactants. RSC Adv. 2015, 5, 102462–102468. [Google Scholar] [CrossRef]
- Dong, R.X.; Liu, C.T.; Huang, K.C.; Chiu, W.Y.; Ho, K.C.; Lin, J.J. Controlling formation of silver/carbon nanotube networks for highly conductive film surface. ACS Appl. Mater. Interfaces 2012, 4, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.; Kim, D.; Kim, J.S.; Lim, S.; Moon, J. Ink-jet printing of Cu−Ag-based highly conductive tracks on a transparent substrate. Langmuir 2009, 25, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Moon, K.S.; Hua, F.; Wong, C.P. Synthesis and thermal and wetting properties of tin/silver alloy nanoparticles for low melting point lead-free solders. Chem. Mater. 2007, 19, 4482–4485. [Google Scholar] [CrossRef]
- Ma, P.C.; Tang, B.Z.; Kim, J.K. Effect of CNT decoration with silver nanoparticles on electrical conductivity of CNT-polymer composite. Carbon 2008, 46, 1497–1505. [Google Scholar] [CrossRef]
- Shibata, J.; Shimizu, K.I.; Takada, Y.; Shichi, A.; Yoshida, H.; Satokawa, S.; Satsuma, A.; Hattori, T. Structure of active Ag clusters in Ag zeolites for SCR of NO by propane in the presence of hydrogen. J. Catal. 2004, 227, 367–374. [Google Scholar] [CrossRef]
- Aihara, N.; Torigoe, K.; Esumi, K. Preparation and characterization of gold and silver nanoparticles in layered laponite suspensions. Langmuir 1998, 14, 4945–4949. [Google Scholar] [CrossRef]
- Liu, J.; Lee, J.B.; Kim, D.H.; Kim, Y. Preparation of high concentration of silver colloidal nanoparticles in layered laponite sol. Colloids Surf. A 2007, 302, 276–279. [Google Scholar] [CrossRef]
- Chiu, C.W.; Hong, P.D.; Lin, J.J. Clay-mediated synthesis of silver nanoparticles exhibiting low-temperature melting. Langmuir 2011, 27, 11690–11696. [Google Scholar] [CrossRef]
- Chiu, C.W.; Huang, T.K.; Wang, Y.C.; Alamani, B.G.; Lin, J.J. Intercalation strategies in clay/polymer hybrids. Prog. Polym. Sci. 2014, 39, 443–485. [Google Scholar] [CrossRef]
- Chiu, C.W.; Chu, C.C.; Dai, S.A.; Lin, J.J. Self-piling silicate rods and dendrites from high aspect-ratio clay platelets. J. Phys. Chem. C 2008, 112, 17940–17944. [Google Scholar] [CrossRef]
- Chiu, C.W.; Lin, J.J. Self-assembly behavior of polymer-assisted clay. Prog. Polym. Sci. 2012, 37, 406–444. [Google Scholar] [CrossRef]
- Koo, J.H.; Jeong, S.; Shim, H.J.; Son, D.; Kim, J.; Kim, D.C.; Choi, S.; Hong, J.I.; Kim, D.H. Wearable electrocardiogram monitor using carbon nanotube electronics and color-tunable organic light-emitting diodes. ACS Nano 2017, 11, 10032–10041. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kim, T.Y.; Park, H.K.; You, I.; Kwak, J.; Kim, J.C.; Hwang, H.; Kim, H.S.; Jeong, U. Hygroscopic auxetic on-skin sensors for easy-to-handle repeated daily use. ACS Appl. Mater. Interfaces 2018, 10, 40141–40148. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Sample | Weight Fraction (w/w/w) | AgNP a (wt%) | Sheet Resistance (Ω/sq) b | ||
|---|---|---|---|---|---|
| 160 °C | 250 °C | 300 °C | |||
| NSP | -- | -- | -- | -- | 1.94 × 106 |
| POE-imide/AgNO3 | 1:1 | -- | -- | 1.45 × 10−1 | 2.68 × 10−2 |
| NSP/POE-imide/AgNO3 | 1:10:10 | 1 | 8.27 × 101 | 6.26 × 10−2 | 5.27 × 10−2 |
| 1:20:20 | 1 | 2.74 × 106 | 2.34 × 103 | 5.32 × 10−2 | |
| 1:10:20 | 1 | -- | 4.45 × 10−2 | 5.56 × 10−2 | |
| 1:10:35 | 5 | 2.76 × 103 | 4.12 × 10−4 | 8.24 × 10−4 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.-Y.; Chiu, C.-W.; Huang, C.-Y.; Shen, S.-Y.; Lee, Y.-C.; Cheng, C.-C.; Jeng, R.-J.; Lin, J.-J. Facile Fabrication of Flexible Electrodes and Immobilization of Silver Nanoparticles on Nanoscale Silicate Platelets to Form Highly Conductive Nanohybrid Films for Wearable Electronic Devices. Nanomaterials 2020, 10, 65. https://doi.org/10.3390/nano10010065
Huang P-Y, Chiu C-W, Huang C-Y, Shen S-Y, Lee Y-C, Cheng C-C, Jeng R-J, Lin J-J. Facile Fabrication of Flexible Electrodes and Immobilization of Silver Nanoparticles on Nanoscale Silicate Platelets to Form Highly Conductive Nanohybrid Films for Wearable Electronic Devices. Nanomaterials. 2020; 10(1):65. https://doi.org/10.3390/nano10010065
Chicago/Turabian StyleHuang, Peng-Yang, Chih-Wei Chiu, Chen-Yang Huang, Sheng-Yen Shen, Yen-Chen Lee, Chih-Chia Cheng, Ru-Jong Jeng, and Jiang-Jen Lin. 2020. "Facile Fabrication of Flexible Electrodes and Immobilization of Silver Nanoparticles on Nanoscale Silicate Platelets to Form Highly Conductive Nanohybrid Films for Wearable Electronic Devices" Nanomaterials 10, no. 1: 65. https://doi.org/10.3390/nano10010065
APA StyleHuang, P. -Y., Chiu, C. -W., Huang, C. -Y., Shen, S. -Y., Lee, Y. -C., Cheng, C. -C., Jeng, R. -J., & Lin, J. -J. (2020). Facile Fabrication of Flexible Electrodes and Immobilization of Silver Nanoparticles on Nanoscale Silicate Platelets to Form Highly Conductive Nanohybrid Films for Wearable Electronic Devices. Nanomaterials, 10(1), 65. https://doi.org/10.3390/nano10010065