Fabrication of Metastable Crystalline Nanocomposites by Flash Annealing of Cu47.5Zr47.5Al5 Metallic Glass Using Joule Heating
Abstract
:1. Introduction
2. Materials and Methods
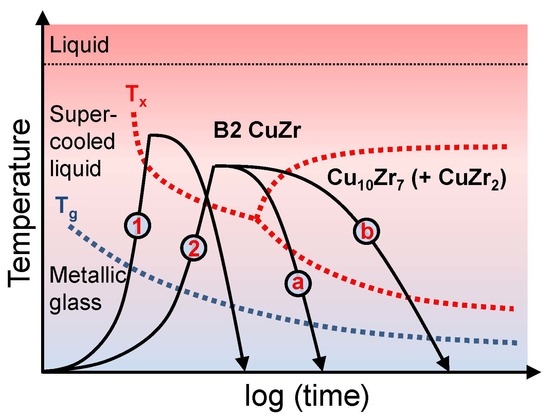
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miracle, D.B.; Donaldson, S.L. ASM Handbook: Composite. In ASM Handbook; ASM International: Material Park, OH, USA, 2001; ISBN 9780871707031. [Google Scholar]
- Skrotzki, W.; Eschke, A.; Romberg, J.; Scharnweber, J.; Marr, T.; Petters, R.; Okulov, I.; Oertel, C.-G.; Freudenberger, J.; Kühn, U.; et al. Processing of High Strength Light-Weight Metallic Composites. Adv. Eng. Mater. 2014, 16, 1208–1216. [Google Scholar] [CrossRef]
- Okulov, I.V.; Weissmüller, J.; Markmann, J. Dealloying-based interpenetrating-phase nanocomposites matching the elastic behavior of human bone. Sci. Rep. 2017, 7, 20. [Google Scholar] [CrossRef]
- Marr, T.; Freudenberger, J.; Seifert, D.; Klauß, H.; Romberg, J.; Okulov, I.; Scharnweber, J.; Eschke, A.; Oertel, C.-G.; Skrotzki, W.; et al. Ti-Al Composite Wires with High Specific Strength. Metals 2011, 1, 79–97. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Kobler, A.; Kübel, C.; Jelitto, H.; Schneider, G.; Weissmüller, J. Nanoporous-gold-based composites: Toward tensile ductility. NPG Asia Mater. 2015, 7, 187. [Google Scholar] [CrossRef] [Green Version]
- Marr, T.; Freudenberger, J.; Kauffmann, A.; Romberg, J.; Okulov, I.; Petters, R.; Scharnweber, J.; Eschke, A.; Oertel, C.-G.; Kühn, U.; et al. Processing of intermetallic titanium aluminide wires. Metals 2013, 3, 188–201. [Google Scholar] [CrossRef] [Green Version]
- Okulov, I.V.; Bönisch, M.; Volegov, A.S.; Shahabi Shakur, H.; Wendrock, H.; Gemming, T.; Eckert, J. Micro-to-nano-scale deformation mechanism of a Ti-based dendritic-ultrafine eutectic alloy exhibiting large tensile ductility. Mater. Sci. Eng. A 2017, 682, 673–678. [Google Scholar] [CrossRef]
- Yoshizawa, Y.; Oguma, S.; Yamauchi, K. New Fe-based soft magnetic alloys composed of ultrafine grain structure. J. Appl. Phys. 1988, 64, 6044. [Google Scholar] [CrossRef]
- Hofmann, D.C.; Suh, J.-Y.; Wiest, A.; Duan, G.; Lind, M.-L.; Demetriou, M.D.; Johnson, W.L. Designing metallic glass matrix composites with high toughness and tensile ductility. Nature 2008, 451, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Okulov, I.V.; Geslin, P.-A.; Soldatov, I.V.; Ovri, H.; Joo, S.-H.; Kato, H. Anomalously low modulus of the interpenetrating-phase composite of Fe and Mg obtained by liquid metal dealloying. Scr. Mater. 2019, 163, 133–136. [Google Scholar] [CrossRef]
- Okulov, A.V.; Volegov, A.S.; Weissmüller, J.; Markmann, J.; Okulov, I.V. Dealloying-based metal-polymer composites for biomedical applications. Scr. Mater. 2018, 146, 290–294. [Google Scholar] [CrossRef]
- Okulov, I.V.; Okulov, A.V.; Volegov, A.S.; Markmann, J. Tuning microstructure and mechanical properties of open porous TiNb and TiFe alloys by optimization of dealloying parameters. Scr. Mater. 2018, 154, 68–72. [Google Scholar] [CrossRef]
- Wang, K.; Weissmüller, J. Composites of nanoporous gold and polymer. Adv. Mater. 2013, 25, 1280–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, T.; Yubuta, K.; Inoue, A.; Kato, H. Dealloying by metallic melt. Mater. Lett. 2011, 65, 1076–1078. [Google Scholar] [CrossRef]
- Okulov, I.V.; Lamaka, S.V.; Wada, T.; Yubuta, K.; Zheludkevich, M.L.; Weissmüller, J.; Markmann, J.; Kato, H. Nanoporous magnesium. Nano Res. 2018, 11, 6428–6435. [Google Scholar] [CrossRef]
- Joo, S.-H.; Bae, J.W.; Park, W.-Y.; Shimada, Y.; Wada, T.; Kim, H.S.; Takeuchi, A.; Konno, T.J.; Kato, H.; Okulov, I.V. Beating Thermal Coarsening in Nanoporous Materials via High-Entropy Design. Adv. Mater. 2019. [Google Scholar] [CrossRef]
- Okulov, I.V.; Okulov, A.V.; Soldatov, I.V.; Luthringer, B.; Willumeit-Römer, R.; Wada, T.; Kato, H.; Weissmüller, J.; Markmann, J. Open porous dealloying-based biomaterials as a novel biomaterial platform. Mater. Sci. Eng. C 2018, 83, 95–103. [Google Scholar] [CrossRef]
- Shi, S.; Markmann, J.; Weissmüller, J. Actuation by hydrogen electrosorption in hierarchical nanoporous palladium. Philos. Mag. 2017, 97, 1571–1587. [Google Scholar] [CrossRef]
- Lührs, L.; Soyarslan, C.; Markmann, J.; Bargmann, S.; Weissmüller, J. Elastic and plastic Poisson’s ratios of nanoporous gold. Scr. Mater. 2016, 110, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Okulov, I.V.; Soldatov, I.V.; Sarmanova, M.F.; Kaban, I.; Gemming, T.; Edström, K.; Eckert, J. Flash Joule heating for ductilization of metallic glasses. Nat. Commun. 2015, 6, 7932. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Inoue, A. Nano-Devitrification of Glassy Alloys. J. Nanosci. Nanotechnol. 2005, 5, 999–1014. [Google Scholar] [CrossRef]
- Okulov, I.V.; Sarmanova, M.F.; Volegov, A.S.; Okulov, A.; Kühn, U.; Skrotzki, W.; Eckert, J. Effect of boron on microstructure and mechanical properties of multicomponent titanium alloys. Mater. Lett. 2015, 158, 111–114. [Google Scholar] [CrossRef]
- Okulov, I.V.; Kühn, U.; Marr, T.; Freudenberger, J.; Soldatov, I.V.; Schultz, L.; Oertel, C.-G.; Skrotzki, W.; Eckert, J. Microstructure and mechanical properties of new composite structured Ti–V–Al–Cu–Ni alloys for spring applications. Mater. Sci. Eng. A 2014, 603, 76–83. [Google Scholar] [CrossRef]
- Okulov, I.V.; Kühn, U.; Romberg, J.; Soldatov, I.V.; Freudenberger, J.; Schultz, L.; Eschke, A.; Oertel, C.-G.; Skrotzki, W.; Eckert, J. Mechanical behavior and tensile/compressive strength asymmetry of ultrafine structured Ti–Nb–Ni–Co–Al alloys with bi-modal grain size distribution. Mater. Des. 2014, 62, 14–20. [Google Scholar] [CrossRef]
- Okulov, I.V.; Bönisch, M.; Okulov, A.V.; Volegov, A.S.; Attar, H.; Ehtemam-Haghighi, S.; Calin, M.; Wang, Z.; Hohenwarter, A.; Kaban, I.; et al. Phase formation, microstructure and deformation behavior of heavily alloyed TiNb- and TiV-based titanium alloys. Mater. Sci. Eng. A 2018, 733, 80–86. [Google Scholar] [CrossRef]
- Louzguine, D.V.; Kato, H.; Inoue, A. High strength and ductile bulk Ti–Ni–Cu–Nb alloy with submicron-size structure units obtained by arc-melting. J. Alloy. Compd. 2004, 375, 171–174. [Google Scholar] [CrossRef]
- Okulov, I.V.; Volegov, A.S.; Attar, H.; Bönisch, M.; Calin, M.; Eckert, J. Composition optimization of low modulus and high-strength TiNb-based alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2017, 65, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Okulov, I.V.; Pauly, S.; Kühn, U.; Gargarella, P.; Marr, T.; Freudenberger, J.; Schultz, L.; Scharnweber, J.; Oertel, C.-G.; Skrotzki, W.; et al. Effect of microstructure on the mechanical properties of as-cast Ti–Nb–Al–Cu–Ni alloys for biomedical application. Mater. Sci. Eng. C 2013, 33, 4795–4801. [Google Scholar] [CrossRef]
- Orava, J.; Kaban, I.; Benkocka, M.; Han, X.; Soldatov, I.; Greer, A.L. Fast-heating-induced formation of metallic-glass/crystal composites with enhanced plasticity. Thermochim. Acta 2019, 677, 198–205. [Google Scholar] [CrossRef]
- Allia, P.; Baricco, M.; Knobel, M.; Vinai, F. Soft nanocrystalline ferromagnetic alloys with improved ductility obtained through dc Joule heating of amorphous ribbons. J. Magn. Magn. Mater. 1994, 133, 243–247. [Google Scholar] [CrossRef]
- Allia, P.; Tiberto, P.; Baricco, M.; Vinai, F. dc Joule heating of amorphous metallic ribbons: Experimental aspects and model. Rev. Sci. Instrum. 1993, 64, 1053. [Google Scholar] [CrossRef]
- Allia, P.; Tiberto, P.; Baricco, M.; Vinai, F. Improved ductility of nanocrystalline Fe73.5Nb3Cu1Si13.5B9 obtained by direct-current joule heating. Appl. Phys. Lett. 1993, 63, 2759. [Google Scholar] [CrossRef]
- Kosiba, K.; Scudino, S.; Kobold, R.; Kühn, U.; Greer, A.L.; Eckert, J.; Pauly, S. Transient nucleation and microstructural design in flash-annealed bulk metallic glasses. Acta Mater. 2017, 127, 416–425. [Google Scholar] [CrossRef]
- Kosiba, K.; Pauly, S. Inductive flash-annealing of bulk metallic glasses. Sci. Rep. 2017, 7, 2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, K.K.; Han, X.L.; Pauly, S.; Qin, Y.S.; Kosiba, K.; Peng, C.X.; Gong, J.H.; Chen, P.X.; Wang, L.; Sarac, B.; et al. Rapid and partial crystallization to design ductile CuZr-based bulk metallic glass composites. Mater. Des. 2018, 139, 132–140. [Google Scholar] [CrossRef]
- Smili, B.; Messaoud, A.; Bouchelaghem, W.; Abadlia, L.; Fazel, N.; Benmoussa, A.; Kaban, I.; Gasser, F.; Gasser, J.G. Temperature dependence of the electrical resistivity and absolute thermoelectric power of amorphous metallic glass Ni33.3Zr66.7. J. Non. Cryst. Solids 2018, 481, 352–360. [Google Scholar] [CrossRef]
- Kaban, I.; Khalouk, K.; Gasser, F.; Gasser, J.-G.; Bednarčik, J.; Shuleshova, O.; Okulov, I.; Gemming, T.; Mattern, N.; Eckert, J. In situ studies of temperature-dependent behaviour and crystallisation of Ni36.5Pd36.5P27 metallic glass. J. Alloy. Compd. 2014, 615, S208–S212. [Google Scholar] [CrossRef]
- Pauly, S.; Das, J.; Mattern, N.; Kim, D.H.; Eckert, J. Phase formation and thermal stability in Cu–Zr–Ti(Al) metallic glasses. Intermetallics 2009, 17, 453–462. [Google Scholar] [CrossRef]
- Tretyachenko, L. Al-Cu-Zr (Aluminium-Copper-Zirconium). In Light Metal Ternary Systems: Phase Diagrams, Crystallographic and Thermodynamic Data; Effenberg, G., Ilyenko, S., Eds.; Landolt-Börnstein—Group IV Physical Chemistry; SpringerMaterials—The Landolt-Börnstein Database; Springer: Berlin/Heidelberg, Germany, 2014; Volume 11A2, pp. 206–223. [Google Scholar]
- Kaban, I.; Jóvári, P.; Escher, B.; Tran, D.T.; Svensson, G.; Webb, M.A.; Regier, T.Z.; Kokotin, V.; Beuneu, B.; Gemming, T.; et al. Atomic structure and formation of CuZrAl bulk metallic glasses and composites. Acta Mater. 2015, 100, 369–376. [Google Scholar] [CrossRef]
- Callister, W.D. Materials Science and Engineering: An Introduction; John Wiley & Sons, Inc.: New York, NY, USA, 2007. [Google Scholar]





| Sample | Current Density (MA m−2) | Estimated Heating Rate (K s−1) | Annealing Time | Volume Fraction of B2 CuZr (vol.%) | Volume Fraction of Cu10Zr7 (vol.%) | Number of Cu10Zr7 Particles (mm−2) | Size of Cu10Zr7 Particles (µm) |
|---|---|---|---|---|---|---|---|
| B2-98 | 59 ± 5 | ≥830 | Until resistivity drop | 98 ± 1 | 2 ± 1 | 1.1 × 104 ± 0.1 | 2.3 ± 0.3 |
| B2-83 | 44 ± 5 | ≥330 | 83 ± 3 | 17 ± 3 | 22.0 × 104 ± 0.7 | 1.7 ± 0.2 | |
| B2-59 | 34 ± 5 | ≥150 | 59 ± 5 | 41 ± 5 | 61.2 × 104 ± 1.5 | 1.1 ± 0.3 | |
| B2-27 | 34 ± 5 | ≥150 | 1.6 s after resistivity drop | 27 ± 4 | 73 ± 4 | 146.9 × 104 ± 2.8 | 1.2 ± 0.2 |
| B2-11 | 34 ± 5 | ≥150 | 2.2 s after resistivity drop | 11 ± 3 | 89 ± 3 | 169.5 × 104 ± 2.1 | 1.0 ± 0.2 |
| Sample | Yield Strength (MPa) | Ultimate Tensile Strength (MPa) | Young’s Modulus (GPa) | Strain to Fracture (%) |
|---|---|---|---|---|
| B2-27 | 1440 ± 30 | 1580 ± 50 | 94.9 ± 0.6 | 1.8 ± 0.2 |
| B2-59 | 1220 ± 30 | 1670 ± 50 | 94.3 ± 0.4 | 2.7 ± 0.1 |
| B2-83 | 980 ± 30 | 1710 ± 50 | 87.2 ± 0.4 | 7.5 ± 0.5 |
| B2-98 | 700 ± 30 | 1320 ± 50 | 79.5 ± 0.8 | 7.1 ± 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okulov, I.; Soldatov, I.; Kaban, I.; Sarac, B.; Spieckermann, F.; Eckert, J. Fabrication of Metastable Crystalline Nanocomposites by Flash Annealing of Cu47.5Zr47.5Al5 Metallic Glass Using Joule Heating. Nanomaterials 2020, 10, 84. https://doi.org/10.3390/nano10010084
Okulov I, Soldatov I, Kaban I, Sarac B, Spieckermann F, Eckert J. Fabrication of Metastable Crystalline Nanocomposites by Flash Annealing of Cu47.5Zr47.5Al5 Metallic Glass Using Joule Heating. Nanomaterials. 2020; 10(1):84. https://doi.org/10.3390/nano10010084
Chicago/Turabian StyleOkulov, Ilya, Ivan Soldatov, Ivan Kaban, Baran Sarac, Florian Spieckermann, and Jürgen Eckert. 2020. "Fabrication of Metastable Crystalline Nanocomposites by Flash Annealing of Cu47.5Zr47.5Al5 Metallic Glass Using Joule Heating" Nanomaterials 10, no. 1: 84. https://doi.org/10.3390/nano10010084
APA StyleOkulov, I., Soldatov, I., Kaban, I., Sarac, B., Spieckermann, F., & Eckert, J. (2020). Fabrication of Metastable Crystalline Nanocomposites by Flash Annealing of Cu47.5Zr47.5Al5 Metallic Glass Using Joule Heating. Nanomaterials, 10(1), 84. https://doi.org/10.3390/nano10010084