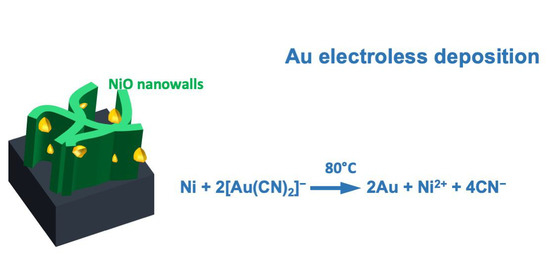
Role of Substrate in Au Nanoparticle Decoration by Electroless Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dai, S.; Chou, J.P.; Wang, K.W.; Hsu, Y.Y.; Hu, A.; Pan, X.; Chen, T.Y. Platinum-trimer decorated cobalt-palladium core-shell nanocatalyst with promising performance for oxygen reduction reaction. Nat. Commun. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranjan, P.; Shankar, S.; Popovitz-Biro, R.; Cohen, S.R.; Kaplan-Ashiri, I.; Dadosh, T.; Shimon, L.J.W.; Višić, B.; Tenne, R.; Lahav, M.; et al. Decoration of Inorganic Nanostructures by Metallic Nanoparticles to Induce Fluorescence, Enhance Solubility, and Tune Band Gap. J. Phys. Chem. C 2018, 122, 6748–6759. [Google Scholar] [CrossRef]
- Su, S.; Xu, Y.; Sun, Q.; Gu, X.; Weng, L.; Wang, L. Noble metal nanostructures-decorated molybdenum disulfide nanocomposites: Synthesis and application. J. Mater. Chem. B 2018, 6, 5323–5334. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Satpati, B.; Mohapatra, S. Plasmon-enhanced photoluminescence from SnO2 nanostructures decorated with Au nanoparticles. Appl. Surf. Sci. 2020, 504, 144381. [Google Scholar] [CrossRef]
- Wang, C.; Wang, T.; Wang, B.; Zhou, X.; Cheng, X.; Sun, P.; Zheng, J.; Lu, G. Design of α-Fe 2 O 3 nanorods functionalized tubular NiO nanostructure for discriminating toluene molecules. Sci. Rep. 2016. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Huang, J.K.; Jin, L.; Hsu, Y.T.; Yu, S.F.; Li, L.J.; Yang, H.Y. Selective decoration of Au nanoparticles on monolayer MoS 2 single crystals. Sci. Rep. 2013. [Google Scholar] [CrossRef]
- Walters, G.; Parkin, I.P. The incorporation of noble metal nanoparticles into host matrix thin films: Synthesis, characterisation and applications. J. Mater. Chem. 2009, 19, 574–590. [Google Scholar] [CrossRef]
- Shang, L.; Zeng, B.; Zhao, F. Fabrication of novel nitrogen-doped graphene-hollow AuPd nanoparticle hybrid films for the highly efficient electrocatalytic reduction of H2O2. ACS Appl. Mater. Interfaces 2015, 7, 122–128. [Google Scholar] [CrossRef]
- Safavi, A.; Farjami, F. Electrodeposition of gold-platinum alloy nanoparticles on ionic liquid-chitosan composite film and its application in fabricating an amperometric cholesterol biosensor. Biosens. Bioelectron. 2011. [Google Scholar] [CrossRef]
- Colombelli, A.; Manera, M.G.; Taurino, A.; Catalano, M.; Convertino, A.; Rella, R. Au nanoparticles decoration of silica nanowires for improved optical bio-sensing. Sens. Actuators B Chem. 2016, 226, 589–597. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Gulina, L.B.; Cho, B.K.; Han, S.H.; Tolstoy, V.P. SnO2-Au nanocomposite synthesized by successive ionic layer deposition method: Characterization and application in gas sensors. Mater. Chem. Phys. 2011, 128, 433–441. [Google Scholar] [CrossRef]
- Hall, D.S.; Lockwood, D.J.; Bock, C.; MacDougall, B.R. Nickel hydroxides and related materials: A review of their structures, synthesis and properties. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015. [Google Scholar] [CrossRef]
- Kim, S.I.; Thiyagarajan, P.; Jang, J.H. Great improvement in pseudocapacitor properties of nickel hydroxide via simple gold deposition. Nanoscale 2014, 6, 11646–11652. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Hu, L.; Chen, Y.; Li, C.; Li, Q.; Wang, Y.; Wei, W.; Chen, L.; Wang, T. Rational design of Au-NiO hierarchical structures with enhanced rate performance for supercapacitors. J. Mater. Chem. A 2013, 1, 7023–7026. [Google Scholar] [CrossRef]
- Urso, M.; Pellegrino, G.; Strano, V.; Bruno, E.; Priolo, F.; Mirabella, S. Enhanced sensitivity in non-enzymatic glucose detection by improved growth kinetics of Ni-based nanostructures. Nanotechnology 2018, 29, 165601. [Google Scholar] [CrossRef]
- Gostin, E.L.; Swan, S.D. Method and Composition for Plating By Chemical Reduction. U.S. Patent 3,032,436, 1 May 1962. [Google Scholar]
- Ali, H.O.; Christie, I.R.A. A review of electroless gold deposition processes. Gold Bull. 1984, 17, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Mallory, G.O.; Hajdu, J.B. Electroless Plating: Fundamentals and Applications; American Electroplaters and Surface Finishers Society: Orlando, FL, USA, 1990. [Google Scholar]
- Stojan, D. Electrodeposition—Theory and Practice. In Modern Aspects of Electrochemistry; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Azmah Hanim, M.A. Electroless Plating as Surface Finishing in Electronic Packaging. In Comprehensive Materials Finishing; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128032503. [Google Scholar]
- Sudagar, J.; Lian, J.; Sha, W. Electroless nickel, alloy, composite and nano coatings—A critical review. J. Alloys Compd. 2013, 571, 183–204. [Google Scholar] [CrossRef] [Green Version]
- Ogata, Y.H.; Kobayashi, K.; Motoyama, M. Electrochemical metal deposition on silicon. Curr. Opin. Solid State Mater. Sci. 2006, 10, 163–172. [Google Scholar] [CrossRef]
- Yae, S.; Nasu, N.; Matsumoto, K.; Hagihara, T.; Fukumuro, N.; Matsuda, H. Nucleation behavior in electroless displacement deposition of metals on silicon from hydrofluoric acid solutions. Electrochim. Acta 2007. [Google Scholar] [CrossRef]
- Ahmad, R.; Bedük, T.; Majhi, S.M.; Salama, K.N. One-step synthesis and decoration of nickel oxide nanosheets with gold nanoparticles by reduction method for hydrazine sensing application. Sens. Actuators B Chem. 2019, 286, 139–147. [Google Scholar] [CrossRef]
- Urso, M.; Tumino, S.; Bruno, E.; Bordonaro, S.; Marletta, D.; Loria, G.R.; Avni, A.; Sacham-Diamand, Y.; Priolo, F.; Mirabella, S. Ultrasensitive Electrochemical Impedance Detection of Mycoplasma agalactiae DNA by Low-Cost and Disposable Au-Decorated NiO Nanowall Electrodes. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef] [PubMed]
- Bahariqushchi, R.; Cosentino, S.; Scuderi, M.; Dumons, E.; Tran-Huu-Hue, L.P.; Strano, V.; Grandjean, D.; Lievens, P.; Poulin-Vittrant, G.; Spinella, C.; et al. Free carrier enhanced depletion in ZnO nanorods decorated with bimetallic AuPt nanoclusters. Nanoscale 2020, 12, 19213–19222. [Google Scholar] [CrossRef]
- Ponnuvelu, D.V.; Dhakshinamoorthy, J.; Prasad, A.K.; Dhara, S.; Kamruddin, M.; Pullithadathil, B. Geometrically Controlled Au-Decorated ZnO Heterojunction Nanostructures for NO2 Detection. ACS Appl. Nano Mater. 2020, 3, 5898–5909. [Google Scholar] [CrossRef]
- Adawiyah, M.A.R.; Azlina, O.S.; Fadil, N.A.; Aisha, S.R.; Hanim, M.A. Electroless and Immersion Plating Process towards Structures and IMC Formation. Int. J. Eng. Technol. 2016, 8, 2558–2570. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, P. Understanding gold plating. Gold Bull. 1986. [Google Scholar] [CrossRef] [Green Version]
- Okinaka, Y.; Hoshino, M. Some recent topics in gold plating for electronics applications. Gold Bull. 1998. [Google Scholar] [CrossRef] [Green Version]
- Vorobyova, T.N.; Poznyak, S.K.; Rimskaya, A.A.; Vrublevskaya, O.N. Electroless gold plating from a hypophosphite-dicyanoaurate bath. Surf. Coatings Technol. 2004, 176, 327–336. [Google Scholar] [CrossRef]
- Iwu, K.O.; Lombardo, A.; Sanz, R.; Scirè, S.; Mirabella, S. Facile synthesis of Ni nanofoam for flexible and low-cost non-enzymatic glucose sensing. Sens. Actuators B Chem. 2016, 224, 764–771. [Google Scholar] [CrossRef]
- Shacham-Diamand, Y.; Inberg, A.; Sverdlov, Y.; Bogush, V.; Croitoru, N.; Moscovich, H.; Freeman, A. Electroless processes for micro- and nanoelectronics. Electrochim. Acta 2003, 48, 2987–2996. [Google Scholar] [CrossRef]
- Li, Z.; Han, C.; Shen, J. Reduction of Ni2+ by hydrazine in solution for the preparation of nickel nano-particles. J. Mater. Sci. 2006, 41, 3473–3480. [Google Scholar] [CrossRef]
- ImageJ. Available online: www.imagej.nih.gov (accessed on 1 October 2019).
- Thompson, M. Xrump. Available online: www.genplot.com (accessed on 1 November 2019).
- Feldman, L.C.; Mayer, J.W.; Grasserbauer, M. Fundamentals of surface and thin film analysis. Anal. Chim. Acta 1987, 199, 288. [Google Scholar] [CrossRef]
- Vratny, F. Electroless Deposition of Gold. U.S. Patent 4,154,877, 24 October 1978. [Google Scholar]
- Liu, H.; Li, N.; Bi, S.; Li, D. Gold immersion deposition on electroless nickel substrates deposition process and influence factor analysis. J. Electrochem. Soc. 2007, 154, D662. [Google Scholar] [CrossRef]
- Bard, A.J.; Parsons, R.; Jordan, J. Standard Potentials in Aqueous Solution; Routledge: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Michaelson, H.B. The work function of the elements and its periodicity. J. Appl. Phys. 1977, 48, 4729–4733. [Google Scholar] [CrossRef] [Green Version]
- Lahiri, A.; Kobayashi, S.I. Electroless deposition of gold on silicon and its potential applications: Review. Surf. Eng. 2016, 32, 321–337. [Google Scholar] [CrossRef]
- Aboelfotoh, M.O.; Cros, A.; Svensson, B.G.; Tu, K.N. Schottky-barrier behavior of copper and copper silicide on n-type and p-type silicon. Phys. Rev. B 1990, 41, 9819–9827. [Google Scholar] [CrossRef]
- Urso, M.; Torrisi, G.; Boninelli, S.; Bongiorno, C.; Priolo, F.; Mirabella, S. Ni(OH)2@Ni core-shell nanochains as low-cost high-rate performance electrode for energy storage applications. Sci. Rep. 2019. [Google Scholar] [CrossRef] [Green Version]
- Lany, S. Semiconducting transition metal oxides. J. Phys. Condens. Matter 2015, 27, 283203. [Google Scholar] [CrossRef]
- Sze, S.M.; Ng, K.K. Physics of Semiconductor Devices, 3rd ed.; Sze, S.M., Ng, K.K., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]








| Sample | Substrate | ELD Bath Immersion Time (s) |
|---|---|---|
| Ni | Ni layer | 30, 60, 90, 120, 150 |
| NiO | NiO layer | 90 |
| Si-n | n-type c-Si | 30, 90, 180 |
| Si-p | p-type c-Si | 30, 90, 180 |
| NiO NWLs | NiO nanowalls | 30, 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, L.; Urso, M.; Shacham-Diamand, Y.; Priolo, F.; Mirabella, S. Role of Substrate in Au Nanoparticle Decoration by Electroless Deposition. Nanomaterials 2020, 10, 2180. https://doi.org/10.3390/nano10112180
Bruno L, Urso M, Shacham-Diamand Y, Priolo F, Mirabella S. Role of Substrate in Au Nanoparticle Decoration by Electroless Deposition. Nanomaterials. 2020; 10(11):2180. https://doi.org/10.3390/nano10112180
Chicago/Turabian StyleBruno, Luca, Mario Urso, Yosi Shacham-Diamand, Francesco Priolo, and Salvo Mirabella. 2020. "Role of Substrate in Au Nanoparticle Decoration by Electroless Deposition" Nanomaterials 10, no. 11: 2180. https://doi.org/10.3390/nano10112180
APA StyleBruno, L., Urso, M., Shacham-Diamand, Y., Priolo, F., & Mirabella, S. (2020). Role of Substrate in Au Nanoparticle Decoration by Electroless Deposition. Nanomaterials, 10(11), 2180. https://doi.org/10.3390/nano10112180