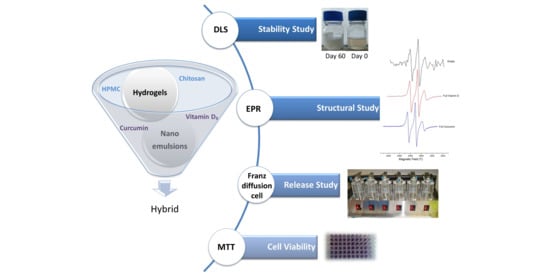
Development and Study of Nanoemulsions and Nanoemulsion-Based Hydrogels for the Encapsulation of Lipophilic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Nanoemulsions
2.2.2. Preparation of Hydrogels
2.2.3. Viscosity Measurements
2.2.4. Dynamic Light Scattering (DLS) Measurements
2.2.5. Stability Study
2.2.6. Electron Paramagnetic Resonance (EPR) Spectroscopy Measurements
2.2.7. In Vitro Release Study
2.2.8. Cell Culture
2.2.9. In Vitro Cell Proliferation Assay
3. Results and Discussion
3.1. Oil-in-Water (o/w) Nanoemulsions
3.1.1. Preparation of Nanoemulsions
3.1.2. Droplet Size and PdI
3.1.3. Stability
3.1.4. Viscosity
3.2. Encapsulation of Lipophilic Compounds in o/w Nanoemulsions
3.2.1. Droplet Size, PdI and Stability
- Vitamin D3
- Curcumin
3.2.2. Dynamics of the Surfactant Layer
- Vitamin D3
- Curcumin
3.3. Nanoemulsion-Based Hydrogels
3.3.1. Preparation of Nanoemulsion-Based Hydrogels
3.3.2. Dynamics of the Surfactant Layer in Nanoemulsion-Based Hydrogels
3.4. In Vitro Release Study
3.5. In Vitro Cell Viability
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akhavan, S.; Assadpour, E.; Katouzian, I.; Jafari, S.M. Lipid nano scale cargos for the protection and delivery of food bioactive ingredients and nutraceuticals. Trends Food Sci. Technol. 2018, 74, 132–146. [Google Scholar] [CrossRef]
- Theochari, I.; Xenakis, A.; Papadimitriou, V. Nanocarriers for effective drug delivery. In Smart Nanocontainers; Tri, P.N., Do, T.-O., Nguyen, T.A., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; Volume 19, pp. 315–341. [Google Scholar]
- Rafiee, Z.; Jafari, S.M. Application of Lipid Nanocarriers for the Food Industry. In Bioactive Molecules in Food; Mérillon, J.M., Ramawat, K.G., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 1–43. [Google Scholar]
- Joey, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Golfomitsou, I.; Mitsou, E.; Xenakis, A.; Papadimitriou, V. Development of Food Grade O/W Nanoemulsions as Carriers of Vitamin D for the Fortification of Emulsion Based Food Matrices: A Structural and Activity Study. J. Mol. Liq. 2018, 268, 734–742. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Soliva-Fortuny, R.C.; Rojas-Graü, M.A.; Martín-Belloso, O.; McClements, D.J. Edible nanoemulsions as carriers of active ingredients: A review. Annu. Rev. Food Sci. Technol. 2017, 8, 439–466. [Google Scholar] [CrossRef]
- Ozturk, B. Nanoemulsions for food fortification with lipophilic vitamins: Production challenges, stability, and bioavailability. Eur. J. Lipid Sci. Technol. 2017, 119, 1500539. [Google Scholar] [CrossRef]
- Tayeb, H.H.; Frank Sainsbury, F. Nanoemulsions in drug delivery: Formulation to medical application. Nanomedicine 2018, 13, 2507–2525. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, structure, and physical properties. J. Phys. Condens. Matter 2006, 18, R635–R666. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [Green Version]
- Komaiko, J.; McClements, D.J. Food-grade nanoemulsion filled hydrogels formed by spontaneous emulsification and gelation: Optical properties, rheology, and stability. Food Hydrocoll. 2015, 46, 67–75. [Google Scholar] [CrossRef]
- Lai, W.F.; Rogach, A.L. Hydrogel-based materials for delivery of herbal medicines. ACS Appl. Mater. Interfaces 2017, 9, 11309–11320. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marafona, P.; Fachel, F.N.S.; Dal Pra, M.; Bassani, V.L.; Koester, L.C.; Henriques, A.T.; Braganhol, E.; Teixeira, H.F. Development, physico-chemical characterization and in-vitro studies of hydrogels containing rosmarinic acid-loaded nanoemulsion for topical application. J. Pharm. Pharm. 2019, 71, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Rostamia, H.; Nikoo, A.M.; Rajabzadeh, G.; Niknia, N.; Salehi, S. Development of cumin essential oil nanoemulsions and its emulsion filled hydrogels. Food Biosci. 2018, 26, 126–132. [Google Scholar] [CrossRef]
- Kelmann, R.G.; Colombo, M.; Nunes, R.J.; Simões, C.M.O.; Koester, L.S. Nanoemulsion-Loaded Hydrogels for Topical Administration of Pentyl Gallate. AAPS PharmSciTech 2018, 19, 2672–2678. [Google Scholar] [CrossRef]
- de Vargas, B.A.; Bidone, J.; Oliveira, L.K.; Koester, L.S.; Bassani, V.L.; Teixeira, H.F. Development of topical hydrogels containing genistein-loaded nanoemulsions. J. Biomed. Nanotechnol. 2012, 8, 330–336. [Google Scholar] [CrossRef]
- Moradi, S.; Barati, A.; Salehi, E.; Tonelli, A.E.; Hamedi, H. Preparation and characterization of chitosan based hydrogels containing cyclodextrin inclusion compounds or nanoemulsions of thyme oil. Polym. Int. 2019, 68, 1891–1902. [Google Scholar] [CrossRef]
- DeLuca, G.C.; Kimball, S.M.; Kolasinski, J.; Ramagopalan, S.V.; Ebers, G.C. Review: The role of vitamin D in nervous system health and disease. Neuropathol. Appl. Neurobiol. 2013, 39, 458–484. [Google Scholar] [CrossRef]
- Gonnet, M.; Lethuaut, L.; Boury, F. New trends in encapsulation of liposoluble vitamins. J. Control Release 2010, 146, 276–290. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. Vitamin D: Evolutionary, Physiological and Health Perspectives. In Current Drug Targets; Bentham Science Publishers: Sharjah, UAE, 2011; Volume 12, pp. 4–18. [Google Scholar]
- Park, S.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Development of nanostructured lipid carriers for the encapsulation and controlled release of vitamin D3. Food Chem. 2017, 225, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Alsaqr, A.; Rasoully, M.; Musteata, F.M. Investigating Transdermal Delivery of Vitamin D3. AAPS PharmSciTech 2015, 16, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Glowka, E.; Stasiak, J.; Lulek, J. Drug Delivery Systems for Vitamin D, Supplementation and Therapy. Pharmaceutics 2019, 11, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonvico, F.; Clementino, A.; Buttini, F.; Colombo, G.; Pescina, S.; Stanisçuaski Guterres, S.; Raffin Pohlmann, A.; Nicoli, S. Surface-Modified Nanocarriers for Nose-to-Brain Delivery: From Bioadhesion to Targeting. Pharmaceutics 2018, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef]
- Nikolic, I.; Mitsou, E.; Damjanovic, A.; Papadimitriou, V.; Stankovic, J.A.; Stanojevic, B.; Xenakis, A.; Savic, S. Curcumin-loaded low-energy nanoemulsions: Linking EPR spectroscopy-analysed microstructure and antioxidant potential with in vitro evaluated biological activity. J. Mol. Liq. 2020, 301, 112479. [Google Scholar] [CrossRef]
- Chen, X.; Zhi, F.; Jia, X.; Zhang, X.; Ambardekar, R.; Meng, Z.; Paradkar, A.R.; Hu, Y.; Yang, Y. Enhanced brain targeting of curcumin by intranasal administration of a thermosensitive poloxamer hydrogel. J. Pharm. Pharmacol. 2013, 65, 807–816. [Google Scholar] [CrossRef]
- Kalaitzaki, A.; Emo, M.; Stébé, M.J.; Xenakis, A.; Papadimitriou, V. Biocompatible nanodispersions as delivery systems of food additives. Food Res. Int. 2013, 54, 1448–1454. [Google Scholar] [CrossRef]
- Griffith, O.H.; Jost, P.C. Lipid Spin Labels in Biological Membrane. In Spin Labeling, Theory and Applications; Berliner, L.J., Ed.; Academic Press: New York, NY, USA, 1976; pp. 454–484. [Google Scholar]
- Kalaitzaki, A.; Poulopoulou, M.; Papadimitriou, V.; Xenakis, A. Surfactant-rich biocompatible microemulsions for transdermal administration of methylxanthine drugs. Colloids Surf. A Physicochem. Eng. Asp. 2014, 442, 80–87. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmed, A. Tween 80 and Soya-Lecithin-Based Food-Grade Nanoemulsions for the Effective Delivery of Vitamin D. Langmuir 2020, 36, 2886–2892. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat Doost, A.; Stevens, C.V.; Claeys, M.; Van Der Meeren, P. Fundamental study on the salt tolerance of oregano essential oil-in-water nanoemulsions containing tween 80. Langmuir 2019, 35, 10572–10581. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzaki, A.; Papanikolaou, N.E.; Karamaouna, F.; Dourtoglou, V.; Xenakis, A.; Papadimitriou, V. Biocompatible Colloidal Dispersions as Potential Formulations of Natural Pyrethrins: A Structural and Efficacy Study. Langmuir 2015, 31, 5722–5730. [Google Scholar] [CrossRef]
- Demisli, S.; Theochari, I.; Christodoulou, P.; Zervou, M.; Xenakis, A.; Papadimitriou, V. Structure, activity and dynamics of extra virgin olive oil-in-water nanoemulsions loaded with vitamin D3 and calcium citrate. J. Mol. Liq. 2020, 306, 112908. [Google Scholar] [CrossRef]
- Ding, Z.; Jiang, Y.; Liu, X. Nanoemulsions-Based Drug Delivery for Brain Tumors. In Nanotechnology-Based Targeted Drug Delivery Systems for Brain Tumors; Kesharwani, P., Gupta, U., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 327–358. [Google Scholar]
- Fanun, M. Microemulsions with mixed Nonionic surfactants. In Microemulsions: Properties and Applications; Fanun, M., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 87–142. [Google Scholar]
- Peng, L.C.; Liu, C.H.; Kwan, C.C.; Huang, K.F. Optimization of water-in-oil nanoemulsions by mixed surfactants, Colloids Surf. A Physicochem. Colloids Surf. A Physicochem. Eng. Asp. 2010, 370, 136–142. [Google Scholar] [CrossRef]
- Klang, V.; Valenta, C. Lecithin-based nanoemulsions. J. Drug. Deliv. Sci. Technol. 2011, 21, 55–76. [Google Scholar] [CrossRef]
- Yuliani, S.; Noveriza, R. Effect of Carrier Oil and Co-Solvent on the Formation of Clove Oil Nanoemulsion by Phase Inversion Technique. Earth Environ. Sci. 2019, 309, 012036. [Google Scholar] [CrossRef] [Green Version]
- Wooster, T.J.; Labbett, D.; Sanguansria, P.; Andrews, H. Impact of microemulsion inspired approaches on the formation and destabilisation mechanisms of triglyceride nanoemulsions. Soft Matter 2016, 12, 1425–1435. [Google Scholar] [CrossRef]
- Gupta, A.; Burak Eral, H.; Hatton, T.A.; Doyle, P.S. Controlling and predicting droplet size of nanoemulsions: Scaling relations with experimental validation. Soft Matter 2016, 12, 1452–1458. [Google Scholar] [CrossRef] [Green Version]
- Nikolic, I.; Lunter, D.J.; Randjelovic, D.; Zugic, A.; Tadic, V.; Markovic, B.; Cekic, N.; Zivkovic, L.; Topalovic, D.; Spremo-Potparevic, B.; et al. Curcumin-loaded low-energy nanoemulsions as a prototype of multifunctional vehicles for different administration routes: Physicochemical and in vitro peculiarities important for dermal application. Int. J. Pharm. 2018, 550, 333–346. [Google Scholar] [CrossRef]
- Avramiotis, S.; Papadimitriou, V.; Hatzara, E.; Bekiari, V.; Lianos, P.; Xenakis, A. Lecithin Organogels Used as Bioactive Compounds Carriers. A Microdomain Properties Investigation. Langmuir 2007, 23, 4438–4447. [Google Scholar] [CrossRef]
- Ilnytskyi, J.; Patsahan, T.; Pizio, O. On the properties of the curcumin molecule in water. Exploration of the OPLS—United atom model by molecular dynamics computer simulation. J. Mol. Liq. 2016, 223, 707–715. [Google Scholar] [CrossRef]
- Hashemnejad, S.M.; Badruddoza, A.Z.M.; Zarket, B.; Castaneda, C.R.; Doyle, P.S. Thermoresponsive nanoemulsion-based gel synthesized through a low-energy process. Nat. Commun. 2019, 10, 2749. [Google Scholar] [CrossRef]
- Montenegro, L.; Carbone, C.; Condorelli, G.; Drago, R.; Puglisi, G. Effect of oil phase lipophilicity on in vitro drug release from o/w microemulsions with low surfactant content. Drug Dev. Ind. Pharm. 2006, 32, 539–548. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Kürti, L.; Veszelka, S.; Bocsik, A.; Dung, N.T.K.; Ózsvári, B.; Puskás, L.G.; Kittel, A.; Szabó-Révész, P.; Delia, M.A. The effect of sucrose esters on a culture model of the nasal barrier. Toxicol. In Vitro 2012, 26, 445–454. [Google Scholar] [CrossRef]
- Mitsou, E.; Pletsa, V.; Sotiroudis, G.T.; Panine, P.; Zoumpanioti, M.; Xenakis, A. Development of a microemulsion for encapsulation and delivery of gallic acid. The role of chitosan. Colloids Surf. B Biointerfaces 2020, 190, 110974. [Google Scholar] [CrossRef]
- Espinoza, L.C.; Silva-Abreu, M.; Clares, B.; Rodríguez-Lagunas, M.J.; Halbaut, L.; Cañas, M.-A.; Calpena, A.C. Formulation Strategies to Improve Nose-to-Brain Delivery of Donepezil. Pharmaceutics 2019, 11, 64. [Google Scholar] [CrossRef] [Green Version]






| Ingredients (% w/w) | System | |||
|---|---|---|---|---|
| L1 | L2 | H1 | H2 | |
| Water | 92 | 93 | 92 | 92 |
| Tween 80 | 2 | 2 | 2 | 4 |
| Labrasol | 2 | 2 | 2 | - |
| Maisine | 1 | 1 | 1 | - |
| Transcutol | 1 | - | 1 | - |
| Lecithin | - | - | - | 0.4 |
| IPM | 2 | 0.4 | 2 | - |
| EVOO | - | 1.6 | - | 3.6 |
| System | Mean Droplet Diameter (nm) | PdI | Viscosity (cP) |
|---|---|---|---|
| L1 | 78.6 ± 0.2 | 0.092 ± 0.003 | 1.24 ± 0.01 |
| L2 | 41.8 ± 0.3 | 0.122 ± 0.007 | 1.31 ± 0.04 |
| H1 | 142.9 ± 4.6 | 0.090 ± 0.013 | 1.33 ± 0.02 |
| H2 | 220.5 ± 4.7 | 0.230 ± 0.007 | 1.50 ± 0.02 |
| System | 5-DSA | 16-DSA | ||||
|---|---|---|---|---|---|---|
| τR (ns) | S | αN (×10−4 T) | τR (ns) | S | αN (×10−4 T) | |
| L1 | 2.73 | 0.16 | 13.8 | 0.36 | 0.02 | 14.8 |
| L1/Vitamin D3 | 3.62 | 0.11 | 13.8 | 0.35 | 0.04 | 14.7 |
| L1/Curcumin | 2.14 | 0.11 | 14.0 | 0.33 | 0.04 | 14.8 |
| L1HG | 2.73 | 0.17 | 13.6 | 0.37 | 0.04 | 14.6 |
| L1HG/VD3 | 3.61 | 0.13 | 13.8 | 0.35 | 0.04 | 14.7 |
| L1HG/Curcumin | 2.23 | 0.11 | 14.0 | 0.36 | 0.04 | 14.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demisli, S.; Mitsou, E.; Pletsa, V.; Xenakis, A.; Papadimitriou, V. Development and Study of Nanoemulsions and Nanoemulsion-Based Hydrogels for the Encapsulation of Lipophilic Compounds. Nanomaterials 2020, 10, 2464. https://doi.org/10.3390/nano10122464
Demisli S, Mitsou E, Pletsa V, Xenakis A, Papadimitriou V. Development and Study of Nanoemulsions and Nanoemulsion-Based Hydrogels for the Encapsulation of Lipophilic Compounds. Nanomaterials. 2020; 10(12):2464. https://doi.org/10.3390/nano10122464
Chicago/Turabian StyleDemisli, Sotiria, Evgenia Mitsou, Vasiliki Pletsa, Aristotelis Xenakis, and Vassiliki Papadimitriou. 2020. "Development and Study of Nanoemulsions and Nanoemulsion-Based Hydrogels for the Encapsulation of Lipophilic Compounds" Nanomaterials 10, no. 12: 2464. https://doi.org/10.3390/nano10122464
APA StyleDemisli, S., Mitsou, E., Pletsa, V., Xenakis, A., & Papadimitriou, V. (2020). Development and Study of Nanoemulsions and Nanoemulsion-Based Hydrogels for the Encapsulation of Lipophilic Compounds. Nanomaterials, 10(12), 2464. https://doi.org/10.3390/nano10122464