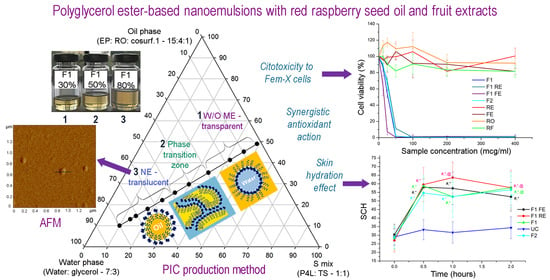
Polyglycerol Ester-Based Low Energy Nanoemulsions with Red Raspberry Seed Oil and Fruit Extracts: Formulation Development toward Effective In Vitro/In Vivo Bioperformance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Nanoemulsion Preparation and Characterization
Phase Inversion Composition (PIC) Method
Particle Size Distribution
Microscopic Investigations
- (a)
- Polarized light microscopy
- (b)
- Atomic force microscopy
Spectroscopic Characterization of Optical Clarity
Electrical Conductivity and pH Value Measurements
Differential Scanning Calorimetry
Continuous Flow (Hysteresis Loop) Test
2.2.2. In Vitro Antioxidant Activity—The DPPH Assay
2.2.3. In Vitro Cytotoxic Activity
Preparation of Stock Solutions
Cell Cultures
Determination of the Target-Cell Survival
2.2.4. In Vivo Safety and Efficacy Assessment
Safety Profile
Moisturizing Efficacy and Skin pH Value
2.3. Statistical Analysis
3. Results and Discussion
3.1. The Optimization of Nanoemulsion Formulations
3.1.1. The Role of Polyglycerol Ester-Based Surfactants
3.1.2. The Role of Cosurfactants, Cosolvents and Red Raspberry Seed Oil
3.2. Characterization of the Transient Phases during the PIC Nanoemulsion Formation and the Final Nanoemulsions
3.2.1. PIC Mechanism of Nanoemulsion Formation
3.2.2. Microscopic Investigations
3.3. Screening of the Nanoemulsion Biological Activity
3.3.1. Preparation and Stability of the Optimized Nanoemulsions
3.3.2. In Vitro Antioxidant Activity
3.3.3. In Vitro Cytotoxic Activity
3.4. In Vivo Safety and Efficacy Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Ethics Committee Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bialek, A.; Bialek, M.; Jelinska, M.; Tokarz, A. Fatty acid profile of new promising unconventional plant oils for cosmetic use. Int. J. Cosmet. Sci. 2016, 38, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Krasodomska, O.; Jungnickel, C. Viability of fruit seed oil O/W emulsions in personal care products. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 468–475. [Google Scholar] [CrossRef]
- Badea, G.; Lacatusu, I.; Badea, N.; Ott, C.; Meghea, A. Use of various vegetable oils in designing photoprotective nanostructured formulations for UV protection and antioxidant activity. Ind. Crops Prod. 2015, 67, 18–24. [Google Scholar] [CrossRef]
- Oomah, B.D.; Ladet, S.; Godfrey, D.V.; Liang, J.; Girard, B. Characteristics of raspberry (Rubus idaeus L.) seed oil. Food Chem. 2000, 69, 187–193. [Google Scholar] [CrossRef]
- Gao, W.; Wang, Y.; Hwang, E.; Lin, P.; Bae, J.; Seo, S.A.; Yan, Z.; Yi, T.-H. Rubus idaeus L. (red raspberry) blocks UVB-induced MMP production and promotes type I procollagen synthesis via inhibition of MAPK/AP-1, NF-κβ and stimulation of TGF-β/Smad, Nrf2 in normal human dermal fibroblasts. J. Photochem. Photobiol. B Biol. 2018, 185, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- McDougall, G.J.; Ross, H.A.; Ikeji, M.; Stewart, D. Berry extracts exert different antiproliferative effects against cervical and colon cancer cells grown in vitro. J. Agric. Food Chem. 2008, 56, 3016–3023. [Google Scholar] [CrossRef]
- Popovic, B.M.; Stajner, D.; Zdero, R.; Orlovic, S.; Galic, Z. Antioxidant characterization of oak extracts combining spectrophotometric assays and chemometrics. Sci. World J. 2013, 2013, 8. [Google Scholar] [CrossRef]
- Gledovic, A.; Janosevic Lezaic, A.; Krstonosic, V.; Djokovic, J.; Nikolic, I.; Bajuk-Bogdanovic, D.; Antic Stankovic, J.; Randjelovic, D.; Savic, S.M.; Filipovic, M.; et al. Low-energy nanoemulsions as carriers for red raspberry seed oil: Formulation approach based on Raman spectroscopy and textural analysis, physicochemical properties, stability and in vitro antioxidant/biological activity. PLoS ONE 2020, 15, e0230993. [Google Scholar] [CrossRef] [Green Version]
- Vinha, A.F.; Costa, A.S.G.; Barreira, J.C.; Pacheco, R.; Oliveira, M.B.P. Chemical and antioxidant profiles of acorn tissues from Quercus spp.: Potential as new industrial raw materials. Ind. Crops Prod. 2016, 94, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical nano and microemulsions for skin delivery. Pharmaceutics 2017, 9, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.A.; Guerreiro, C.M.; Maruno, M.; Ferrari, M.; Rocha-Filho, P.A. Exotic vegetable oils for cosmetic O/W nanoemulsions: In vivo evaluation. Molecules 2016, 21, 248–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An Overview of micro- and nanoemulsions as vehicles for essential Oils: Formulation, preparation and stability. Nanomaterials 2020, 10, 135–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solans, C.; Sole, I. Nano-emulsions: Formation by low-energy methods. Curr. Opin. Colloid Interface Sci. 2012, 17, 246–254. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2016, 8, 1719–1729. [Google Scholar] [CrossRef]
- Le Guenic, S.; Chaveriat, L.; Lequart, V.; Joly, N.; Martin, P. Renewable surfactants for biochemical applications and nanotechnology. J. Surfact. Deterg. 2019, 22, 5–21. [Google Scholar] [CrossRef] [Green Version]
- Hanno, I.; Centini, M.; Anselmi, C.; Bibiani, C. Green cosmetic surfactant from rice: Characterization and application. Cosmetics 2015, 2, 322–341. [Google Scholar] [CrossRef]
- Kato, T.; Nakamura, T.; Yamashita, M.; Kawaguchi, M.; Kato, T.; Itoh, T. Surfactants properties of purified polyglycerol monolaurates. J. Surfact. Deterg. 2003, 6, 331–337. [Google Scholar] [CrossRef]
- Bernard, A.-L.; Ikeda, Y.K.; El Akkari, R.; Simonnet, J.-T. Cosmetic composition. EU Patent 2941238B1, 16 October. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DEP2941238B1 (accessed on 11 December 2020).
- Hameyer, P.; Meyer, J.; Polak, G. Cold-Preparable, Low-Viscosity and Prolonged-Stability Cosmetic Emulsions. U.S. Patent No. 879,569,2B2, 8 May 2008. Available online: https://patents.google.com/patent/US8795692 (accessed on 11 December 2020).
- Sahle, F.F.; Metz, H.; Wohlrab, J.; Neubert, R.H.H. Polyglycerol fatty acid ester surfactant–based microemulsions for targeted delivery of ceramide AP into the stratum corneum: Formulation, characterisation, in vitro release and penetration investigation. Eur. J. Pharm. Biopharm. 2012, 82, 139–150. [Google Scholar] [CrossRef]
- Heunemann, P.; Prevost, S.; Grillo, I.; Marino, C.M.; Meyer, J.; Gradzielski, M. Formation and structure of slightly anionically charged nanoemulsions obtained by the phase inversion concentration (PIC) method. Soft Matter 2011, 7, 5697–5710. [Google Scholar] [CrossRef] [Green Version]
- Wakisaka, S.; Nakanishi, M.; Gohtani, S. Phase behavior and formation of o/w nano-emulsion in vegetable oil/ mixture of polyglycerol polyricinoleate and polyglycerin fatty acid ester/water systems. J. Oleo Sci. 2014, 63, 229–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakisaka, S.; Nishimura, T.; Gohtani, S. O/W nano-emulsion formation using an isothermal low-energy emulsification method in a mixture of polyglycerol polyricinoleate and hexaglycerol monolaurate with glycerol system. J. Oleo Sci. 2015, 64, 405–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Pinagoda, J.; Tupker, R.A.; Agner, T.; Serup, J. Guidelines for transepidermal water loss (TEWL) measurements. Contact Derm. 1990, 22, 164–178. [Google Scholar] [CrossRef]
- Bernardi, D.S.; Pereira, T.A.; Macie, N.R.; Bortoloto, J.; Viera, G.S.; Oliveira, G.C.; Rocha-Filho, P.A. Formation and stability of oil-in-water nanoemulsions containing rice bran oil: In vitro and in vivo assessments. J. Nanobiotechnol. 2011, 9, 44–52. [Google Scholar] [CrossRef]
- Berardesca, E. EEMCO guidance for the assessment of stratum corneum hydration: Electrical methods. Skin Res. Technol. 1997, 3, 126–132. [Google Scholar] [CrossRef]
- Azmi, N.A.N.; Elgharbawy, A.A.M.; Motlagh, S.R.; Samsudin, N.; Salleh, H.M. Nanoemulsions: Factory for Food, Pharmaceutical and Cosmetics. Processes 2019, 7, 617–650. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.Y.; McClements, D.J. Optimization of orange oil nanoemulsion formation by isothermal low-energy methods: Influence of the oil phase, surfactant, and temperature. J. Agric. Food Chem. 2014, 62, 2306–2312. [Google Scholar] [CrossRef]
- Fasolin, L.H.; Santana, R.C.; Cunha, R.L. Microemulsions and liquid crystalline formulated with triacylglycerols: Effect of ethanol and oil unsaturation. Colloids Surf. A 2012, 415, 31–40. [Google Scholar] [CrossRef]
- Johnson, W.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Hill, R.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Final report of the cosmetic ingredient review expert panel on the safety assessment of pelargonic acid (nonanoic acid) and nonanoate esters. Int. J. Toxicol. 2011, 30, 228S–269S. [Google Scholar] [CrossRef] [PubMed]
- Saberi, A.H.; Fang, Y.; McClements, D.J. Stabilization of vitamin E-enriched mini-emulsions: Influence of organic and aqueous phase compositions. Colloids Surf. A Physicochem. Eng. Asp. 2014, 449, 65–73. [Google Scholar] [CrossRef]
- Rocha-Filho, P.A.; Ferrari, M.; Maruno, M.; Souza, O.; Gumiero, V. In vitro and in vivo evaluation of nanoemulsion containing vegetable extracts. Cosmetics 2017, 4, 32–44. [Google Scholar] [CrossRef] [Green Version]
- Do, L.D.; Withayyapayanon, A.; Harwell, J.H.; Sabatini, D.A. Environmentally friendly vegetable oil microemulsions using extended surfactants and linkers. J. Surfact. Deterg. 2009, 12, 91–99. [Google Scholar] [CrossRef]
- Szumala, P. Structure of microemulsion formulated with monoacylglycerols in the presence of polyols and ethanol. J. Surfact. Deterg. 2015, 18, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.M.; Ushiyama, K.; Aramaki, K. Phase behavior, formation, and rheology of cubic phase and related gel emulsion in Tween80/Water/Oil Systems. J. Oleo Sci. 2009, 58, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Nikolic, I.; Lunter, D.J.; Randjelovic, D.; Zugic, A.; Tadic, V.; Markovic, B.; Cekic, N.; Zivkovic, L.; Topalovic, D.; Spremo-Potparevic, B.; et al. Curcumin-loaded low-energy nanoemulsions as a prototype of multifunctional vehicles for different administration routes: Physicochemical and in vitro peculiarities important for dermal application. Int. J. Pharm. 2018, 550, 333–346. [Google Scholar] [CrossRef]
- Savic, V.; Todosijevic, M.; Ilic, T.; Lukic, M.; Mitsou, E.; Papadimitriou, V.; Avramiotis, S.; Markovic, B.; Cekic, N.; Savic, S. Tacrolimus loaded biocompatible lecithin-based microemulsions with improved skin penetration: Structure characterization and in vitro/in vivo performances. Int. J. Pharm. 2017, 529, 491–505. [Google Scholar] [CrossRef]
- Zhang, H.; Taxipalati, M.; Que, F.; Feng, F. Microstructure characterization of a food-grade U-type microemulsion system by differential scanning calorimetry and electrical conductivity techniques. Food Chem. 2013, 141, 3050–3055. [Google Scholar] [CrossRef]
- Hyde, S.T. Identification of lyotropic liquid crystalline mesophases. In Handbook of Applied Surface and Colloid Chemistry; Holberg, K., Ed.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2001; pp. 299–332. [Google Scholar]
- Fonseca-Santos, B.; Satake, C.Y.; Calixto, G.F.M.; Martins dos Santos, A.; Chorilli, M. Trans-resveratrol loaded nonionic lamellar liquid-crystalline systems: Structural, rheological, mechanical, textural, and bioadhesive characterization and evaluation of in vivo anti-inflammatory activity. Int. J. Nanomed. 2017, 12, 6883–6893. [Google Scholar] [CrossRef] [Green Version]
- Alessandrini, A.; Facci, P. Phase transitions in supported lipid bilayers studied by AFM. Soft Matter 2014, 37, 7145–7164. [Google Scholar] [CrossRef]
- Bento da Silva, P.; Fioramonti Calixto, G.M.; Oshiro Junior, J.A.; Avila Bombardelli, R.L.; Fonseca-Santos, B.; Rodero, C.F. Structural features and the anti-inflammatory effect of green tea extract-loaded liquid crystalline systems intended for skin delivery. Polymers 2017, 9, 30–44. [Google Scholar] [CrossRef]
- Rebolleda, S.; Sanz, M.T.; Benito, J.M.; Beltran, S.; Escudero, I.; Gonzalez San-Jose, M.L. Formulation and characterisation of wheat bran oil-in-water nanoemulsions. Food Chem. 2015, 167, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Kulisic, T.; Radonic, A.; Katalinic, V.; Milos, M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Uluata, S.; Ozdemir, N. Antioxidant activities and oxidative stabilities of some unconventional oilseeds. J. Am. Oil. Chem. Soc. 2012, 89, 551–559. [Google Scholar] [CrossRef] [Green Version]
- Francielli de Oliveira, P.; Morais Alves, J.; Lopes Damasceno, J.; Machado Oliveira, R.A.; Dias, H.J.; Miller Crotti, A.E.; Tavares, D.C. Cytotoxicity screening of essential oils in cancer cell lines. Rev. Bras. Farmacogn. 2015, 25, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Siti Syarifah, M.M.; Nurhanan, M.Y.; Muhd Haffiz, J.; Mohd Ilham, A.; Getha, K.; Asiah, O.; Norhayati, I.; Lili Sahira, H.; Anee Suryani, S. Potential anticancer compound from Cerbera Odollam. J. Trop. For. Sci. 2011, 23, 89–96. [Google Scholar]
- Vater, C.; Adamovic, A.; Ruttensteiner, L.; Steiner, K.; Tajpara, P.; Klang, V.; Elbe-Burger, A.; Wirth, M.; Valenta, C. Cytotoxicity of lecithin-based nanoemulsions on human skin cells and ex vivo skin permeation: Comparison to conventional surfactant types. Int. J. Pharm. 2019, 566, 383–390. [Google Scholar] [CrossRef]
- Kapalczynska, M.; Kolenda, T.; Przybyla, W.; Zajaczkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Blizniak, R.; Luczewski, L.; Lamperska, K. 2D and 3D cell cultures–a comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar]
- Nesic, I.; Stojiljkovic, D.; Savic, S.; Tasic-Kostov, M.; Tadic, V. Stability, antioxidant activity, in vivo safety and efficacy of creams with standardized wild apple fruit extract: A comparison of conventional and biodegradable emulsifiers. Int. J. Cosmet. Sci. 2019, 41, 300–310. [Google Scholar] [CrossRef]
- Esposito, E.; Drechsler, M.; Mariani, P.; Sivieri, E.; Bozzini, R.; Montesi, L.; Menegatti, E.; Cortesi, R. Nanosystems for skin hydration: A comparative study. Int. J. Cosmet. Sci. 2007, 29, 39–47. [Google Scholar] [CrossRef] [PubMed]









| Formulation Name | Water Phase wt% (Glycerol: Water) | Oil Phase Phase/Cosurfactant wt% | S Mix wt% | % TP | n-Index | K-Index |
|---|---|---|---|---|---|---|
| F0 30% | 30 (1.5: 28.5) | 35 (7 cosurfactant 2: 28 EP) | 35 | 94.82 ± 0.005 | 0.9895 ± 0.0090 | 0.0532 ± 0.0026 |
| F0 50% | 50 (2.5: 47.5) | 25 (5 cosurfactant 2: 20 EP) | 25 | 87.57 ± 0.006 | 0.9828 ± 0.0026 | 0.0683 ± 0.0008 |
| F0 80% | 80 (4: 76) | 10 (2 cosurfactant 2: 8 EP) | 10 | 82.16 ± 0.037 | 1.0040 ± 0.0019 | 0.0045 ± 0.0004 |
| F1 30% | 30 (9: 21) | 35 (1.75 cosurfactant 1: 7 RO: 26.25 EP) | 35 | 94.42 ± 0.004 | 0.9843 ± 0.0102 | 0.0699 ± 0.0037 |
| F1 50% | 50 (15: 25) | 25 (1.25 cosurfactant 1: 5 RO: 18.75 EP) | 25 | 81.95 ± 0.002 | 0.9938 ± 0.0239 | 0.0617 ± 0.0073 |
| F1 80% | 80 (24: 56) | 10 (0.5 cosurfactant 1: 2 RO: 7.5 EP) | 10 | 76.18 ± 0.019 | 0.9582 ± 0.0136 | 0.0319 ± 0.0022 |
| F2 74% | 74 (22.2: 51.8) | 13 (2.6 cosurfactant 2: 2.6 RO: 7.8 EP) | 13 | 71.12 ± 0.047 | 0.9894 ± 0.0178 | 0.0532 ± 0.0056 |
| F2 80% | 80 (24: 56) | 10 (2 cosurfactant 2: 2 RO: 6 EP) | 10 | 78.10 ± 0.016 | 0.9876 ± 0.0133 | 0.0073 ± 0.0004 |
| Sample Name | Z-Ave (nm) | PDI | pH | El. Cond. |
|---|---|---|---|---|
| (µS/cm) | ||||
| F0 | 24h:131.70 ± 1.114 | 24h: 0.226 ± 0.012 | 24h: 4.56 ± 0.02 | 24h: 128.6 ± 0.20 |
| 3m: 138.30 ± 1.358 | 3m: 0.162 ± 0.023 | 3m: 4.29 ± 0.05 | 3m: 145.4 ± 0.96 | |
| F1 | 24h: 58.21 ± 5.187 | 24h: 0.071 ± 0.015 | 24h: 4.53 ± 0.03 | 24h: 30.68 ± 1.66 |
| 3m: 60.12 ± 8.831 | 3m: 0.099 ± 0.037 | 3m: 4.15 ± 0.01 | 3m: 48.50 ± 0.40 | |
| F1 RE | 24h: 55.48 ± 6.769 | 24h: 0.071 ± 0.010 | 24h: 4.00 ± 0.04 | 24h: 42.73 ± 0.35 |
| 3m: 62.73 ± 1.255 | 3m: 0.079 ± 0.007 | 3m: 3.83 ± 0.01 | 3m: 52.63 ± 0.42 | |
| F1 FE | 24h: 56.37 ± 5.130 | 24h: 0.058 ± 0.024 | 24h: 4.48 ± 0.07 | 24h: 117.53 ± 0.06 |
| 3m: 59.21 ± 10.09 | 3m: 0.089 ± 0.005 | 3m: 4.47 ± 0.15 | 3m: 124.87 ± 0.21 | |
| F2 | 24h: 55.62 ± 1.164 | 24h: 0.093 ± 0.022 | 24h: 4.49 ± 0.28 | 24h: 35.11 ± 4.99 |
| 3m: 59.77 ± 2.208 | 3m: 0.098 ± 0.011 | 3m: 4.29 ± 0.03 | 3m: 41.53 ± 4.46 |
| Sample Name | % INH DPPH | % INH DPPH | ||
|---|---|---|---|---|
| 24 h After Preparation | After 3 Months | |||
| 10 µL/mL | 20 µL/mL | 30 µL/mL | 30 µL/mL | |
| F0 | 0.18 ± 0.03 | 0.55 ± 0.04 | 0.70 ± 0.07 | 1.2 ± 0.15 |
| F1 | 3.73 ± 0.10 | 6.41 ± 0.14 | 8.37 ± 0.55 | 10.19 ± 0.62 |
| F1 RE | 3.91 ± 0.09 | 7.33 ± 0.12 | 9.53 ± 0.41 | 11.74 ± 0.47 |
| F1 FE | 90.87 ± 0.31 | 92.49 ± 0.29 | 91.65 ± 0.93 | 93.79 ± 0.11 |
| F2 | 3.67 ± 0.06 | 8.37 ± 0.07 | 9.90 ± 0.56 | 10.98 ± 0.27 |
| Sample Name | Fem-X | HaCaT | SI * | SI Score ** |
|---|---|---|---|---|
| IC50 (µg/mL) | IC50 (µg/mL) | |||
| F1 | 31.41 ± 8.75 | 164.16 ± 34.57 | 5.23 | 3 |
| F1 RE | 28.99 ± 4.99 | 93.79 ± 42.55 | 3.24 | 2 |
| F1 FE | 25.06 ± 6.58 | 65.06 ± 8.14 | 2.60 | 2 |
| F2 | 29.41 ± 2.52 | 51.05 ± 6.41 | 1.74 | 2 |
| RE | >400 | >400 | NV | NV |
| FE | >400 | >400 | NV | NV |
| RO | >400 | >400 | NV | NV |
| RF | >400 | >400 | NV | NV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gledovic, A.; Janosevic Lezaic, A.; Nikolic, I.; Tasic-Kostov, M.; Antic-Stankovic, J.; Krstonosic, V.; Randjelovic, D.; Bozic, D.; Ilic, D.; Tamburic, S.; et al. Polyglycerol Ester-Based Low Energy Nanoemulsions with Red Raspberry Seed Oil and Fruit Extracts: Formulation Development toward Effective In Vitro/In Vivo Bioperformance. Nanomaterials 2021, 11, 217. https://doi.org/10.3390/nano11010217
Gledovic A, Janosevic Lezaic A, Nikolic I, Tasic-Kostov M, Antic-Stankovic J, Krstonosic V, Randjelovic D, Bozic D, Ilic D, Tamburic S, et al. Polyglycerol Ester-Based Low Energy Nanoemulsions with Red Raspberry Seed Oil and Fruit Extracts: Formulation Development toward Effective In Vitro/In Vivo Bioperformance. Nanomaterials. 2021; 11(1):217. https://doi.org/10.3390/nano11010217
Chicago/Turabian StyleGledovic, Ana, Aleksandra Janosevic Lezaic, Ines Nikolic, Marija Tasic-Kostov, Jelena Antic-Stankovic, Veljko Krstonosic, Danijela Randjelovic, Dragana Bozic, Dusan Ilic, Slobodanka Tamburic, and et al. 2021. "Polyglycerol Ester-Based Low Energy Nanoemulsions with Red Raspberry Seed Oil and Fruit Extracts: Formulation Development toward Effective In Vitro/In Vivo Bioperformance" Nanomaterials 11, no. 1: 217. https://doi.org/10.3390/nano11010217
APA StyleGledovic, A., Janosevic Lezaic, A., Nikolic, I., Tasic-Kostov, M., Antic-Stankovic, J., Krstonosic, V., Randjelovic, D., Bozic, D., Ilic, D., Tamburic, S., & Savic, S. (2021). Polyglycerol Ester-Based Low Energy Nanoemulsions with Red Raspberry Seed Oil and Fruit Extracts: Formulation Development toward Effective In Vitro/In Vivo Bioperformance. Nanomaterials, 11(1), 217. https://doi.org/10.3390/nano11010217