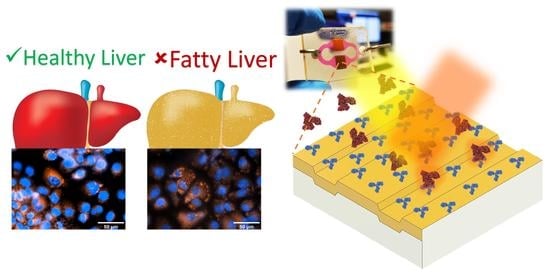
Direct and Label-Free Monitoring of Albumin in 2D Fatty Liver Disease Model Using Plasmonic Nanogratings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Development of the In Vitro Fatty Liver Disease Model
2.2. Fabrication and Integration of the Nanoplasmonic Chip
2.3. Experimental Optical Setup and FDTD Simulations
2.4. Surface Biofunctionalization of Nanoplasmonic Sensing Platform
3. Results and Discussion
3.1. In Vitro Fatty Liver Disease Model
3.2. Simulation and Characterization of Nanoplasmonic Sensor
3.3. Albumin Detection by the Nanoplasmonic Sensing Integrated Platform
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of non-alcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedossa, P. Diagnosis of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: Why liver biopsy is essential. Liver Int. 2018, 38, 64–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Chiara, F.; Checcllo, C.U.; Azcón, J.R. High protein diet and metabolic plasticity in non-alcoholic fatty liver disease: Myths and truths. Nutrients 2019, 11, 2985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Yin, H.; Liu, M.; Xu, G.; Zhou, X.; Ge, P.; Yang, H.; Mao, Y. Impaired albumin function: A novel potential indicator for liver function damage? Ann. Med. 2019, 51, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures – a comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.A.; Estevez, M.C.; Soler, M.; Lechuga, L.M. Recent advances in nanoplasmonic biosensors: Applications and lab-on-a-chip integration. Nanophotonics 2017, 6, 123–136. [Google Scholar] [CrossRef]
- López-Muñoz, G.A.; Estevez, M.C.; Peláez-Gutierrez, E.C.; Homs-Corbera, A.; García-Hernandez, M.C.; Imbaud, J.I.; Lechuga, L.M. A label-free nanostructured plasmonic biosensor based on Blu-ray discs with integrated microfluidics for sensitive biodetection. Biosens. Bioelectron. 2017, 96, 260–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wi, J.S.; Lee, S.; Lee, S.H.; Oh, D.K.; Lee, K.T.; Park, I.; Kwak, M.K.; Ok, J.G. Facile three-dimensional nanoarchitecturing of double-bent gold strips on roll-to-roll nanoimprinted transparent nanogratings for flexible and scalable plasmonic sensors. Nanoscale 2017, 9, 1398–1402. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.L.; Hsu, H.Y.; You, M.L.; Chang, C.C.; Pan, M.Y.; Shi, X.; Ueno, K.; Misawa, H.; Wei, P.K. Highly Sensitive Aluminum-Based Biosensors using Tailorable Fano Resonances in Capped Nanostructures. Sci. Rep. 2017, 7, 44104. [Google Scholar] [CrossRef] [PubMed]
- López-Muñoz, G.A.; Estévez, M.C.; Vázquez-García, M.; Berenguel-Alonso, M.; Alonso-Chamarro, J.; Homs-Corbera, A.; Lechuga, L.M. Gold/silver/gold trilayer films on nanostructured polycarbonate substrates for direct and label-free nanoplasmonic biosensing. J. Biophotonics 2018, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aćimović, S.S.; Ortega, M.A.; Sanz, V.; Berthelot, J.; Garcia-Cordero, J.L.; Renger, J.; Maerkl, S.J.; Kreuzer, M.P.; Quidant, R. LSPR chip for parallel, rapid, and sensitive detection of cancer markers in serum. Nano Lett. 2014, 14, 2636–2641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Chiara, F.; Heebøll, S.; Marrone, G.; Montoliu, C.; Hamilton-Dutoit, S.; Ferrandez, A.; Andreola, F.; Rombouts, K.; Grønbæk, H.; Felipo, V.; et al. Urea cycle dysregulation in non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 905–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estévez, M.-C.; Font, H.; Nichkova, M.; Salvador, J.-P.; Varela, B.; Sánchez-Baeza, F.; Marco, M.-P. Immunochemical Determination of Pharmaceuticals and Personal Care Products as Emerging Pollutants BT—Water Pollution: Emerging Organic Pollution in Waste Waters and Sludge; Barceló, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 2, pp. 181–244. ISBN 978-3-540-31493-6. [Google Scholar]
- Henseleit, A.; Pohl, C.; Bley, T.; Boschke, E. Monitoring human serum albumin cell cultures using surface plasmon resonance (SPR) spectroscopy. JSSS 2015, 4, 77. [Google Scholar] [CrossRef] [Green Version]
- Vashist, S.K.; Saraswat, M.; Holthšfer, H. Comparative study of the developed chemiluminescent, ELISA and SPR immunoassay formats for the highly sensitive detection of human albumin. Procedia Chem. 2012, 6, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Liao, C.; Chen, D.; Song, J.; Jin, W.; Peng, G.D.; Wang, Y. Label-free detection of bovine serum albumin based on an in-fiber Mach-Zehnder interferometric biosensor. Opt. Express 2017, 25, 17105–17113. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.C.; Chang, Y.T.; Kang, Y.T.; Chang, H.Y.; Chang, P.; Yew, T.R. A quantum dot-based optical immunosensor for human serum albumin detection. Biosens. Bioelectron. 2012, 34, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ye, C.; Liu, Z.; Yang, L.; Liu, A.; Zhong, G.; Lin, X. Facilely prepared low-density DNA monolayer–based electrochemical biosensor with high detection performance in human serum. Anal. Bioanal. Chem. 2019, 411, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Ogata, A.F.; Edgar, J.M.; Majumdar, S.; Briggs, J.S.; Patterson, S.V.; Tan, M.X.; Kudlacek, T.S.; Schneider, C.A.; Weiss, G.A.; Penner, R.M. Virus-enabled biosensor for human serum albumin. Anal. Chem. 2017, 89, 1373–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sang, S.; Li, Y.; Guo, X.; Zhang, B.; Xue, X.; Zhuo, K.; Yuan, Z. A portable device for rapid detection of human serum albumin using an immunoglobulin-coating-based magnetoelastic biosensor. Biosens. Bioelectron. 2019, 141, 111399. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Muñoz, G.A.; Ortega, M.A.; Ferret-Miñana, A.; De Chiara, F.; Ramón-Azcón, J. Direct and Label-Free Monitoring of Albumin in 2D Fatty Liver Disease Model Using Plasmonic Nanogratings. Nanomaterials 2020, 10, 2520. https://doi.org/10.3390/nano10122520
Lopez-Muñoz GA, Ortega MA, Ferret-Miñana A, De Chiara F, Ramón-Azcón J. Direct and Label-Free Monitoring of Albumin in 2D Fatty Liver Disease Model Using Plasmonic Nanogratings. Nanomaterials. 2020; 10(12):2520. https://doi.org/10.3390/nano10122520
Chicago/Turabian StyleLopez-Muñoz, Gerardo A., Maria Alejandra Ortega, Ainhoa Ferret-Miñana, Francesco De Chiara, and Javier Ramón-Azcón. 2020. "Direct and Label-Free Monitoring of Albumin in 2D Fatty Liver Disease Model Using Plasmonic Nanogratings" Nanomaterials 10, no. 12: 2520. https://doi.org/10.3390/nano10122520
APA StyleLopez-Muñoz, G. A., Ortega, M. A., Ferret-Miñana, A., De Chiara, F., & Ramón-Azcón, J. (2020). Direct and Label-Free Monitoring of Albumin in 2D Fatty Liver Disease Model Using Plasmonic Nanogratings. Nanomaterials, 10(12), 2520. https://doi.org/10.3390/nano10122520