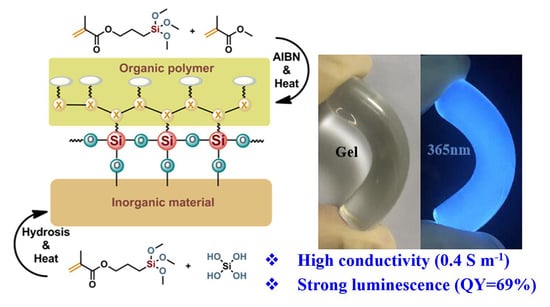
Luminescent Ionogels with Excellent Transparency, High Mechanical Strength, and High Conductivity
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, S.; Sun, J.; Zhang, X.; Xin, J.; Miao, Q.; Wang, J. Ionic liquid-based green processes for energy production. Chem. Soc. Rev. 2014, 43, 7838–7869. [Google Scholar] [CrossRef] [PubMed]
- Scholten, J.D.; Leal, B.C.; Dupont, J. Transition metal nanoparticle catalysis in ionic liquids. ACS Catal. 2011, 2, 184–200. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, T.; Han, B. Preparation of catalytic materials using ionic liquids as the media and functional components. Adv. Mater. 2014, 26, 6810–6827. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Armstrong, D.W. Ionic liquids in separations. Acc. Chem. Res. 2007, 40, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Wang, J.; Wu, K.; Xuan, X.; Lu, X. Ionic liquid-based aqueous two-phase extraction of selected proteins. Sep. Purif. Technol. 2009, 64, 288–295. [Google Scholar] [CrossRef]
- Wu, F.; Chen, N.; Chen, R.; Wang, L.; Li, L. Organically modified silica-supported ionogels electrolyte for high temperature lithium-ion batteries. Nano Energy 2017, 31, 9–18. [Google Scholar] [CrossRef]
- Zhang, L.M.; He, Y.; Cheng, S.; Sheng, H.; Dai, K.; Zheng, W.J.; Wang, M.X.; Chen, Z.S.; Chen, Y.M.; Suo, Z.; et al. Self-healing, adhesive, and highly stretchable ionogel as a strain sensor for extremely large deformation. Small 2019, 15, 1804651. [Google Scholar] [CrossRef]
- Chiappe, C.; Pieraccini, D. Ionic liquids: Solvent properties and organic reactivity. J. Phys. Org. Chem. 2005, 18, 275–297. [Google Scholar] [CrossRef]
- Hapiot, P.; Lagrost, C. Electrochemical reactivity in room-temperature ionic liquids. Chem. Rev. 2008, 108, 2238–2264. [Google Scholar] [CrossRef]
- Visentin, A.F.; Alimena, S.; Panzer, M.J. Influence of ionic liquid selection on the properties of Poly(ethylene glycol) diacrylate-supported ionogels as solid electrolytes. ChemElectroChem 2014, 1, 718–721. [Google Scholar] [CrossRef]
- Xie, Z.L.; Huang, X.; Taubert, A. DyeIonogels: Proton-responsive ionogels based on a dye-ionic liquid exhibiting reversible color change. Adv. Funct. Mater. 2014, 24, 2837–2843. [Google Scholar] [CrossRef]
- Marr, P.C.; Marr, A.C. Ionic liquid gel materials: Applications in green and sustainable chemistry. Green Chem. 2016, 18, 105–128. [Google Scholar] [CrossRef] [Green Version]
- Visentin, A.F.; Dong, T.; Poli, J.; Panzer, M.J. Rapid, microwave-assisted thermal polymerization of poly(ethylene glycol) diacrylate-supported ionogels. J. Mater. Chem. A 2014, 2, 7723. [Google Scholar] [CrossRef] [Green Version]
- Horowitz, A.I.; Panzer, M.J. Poly(dimethylsiloxane)-supported ionogels with a high ionic liquid loading. Angew. Chem. Ent. Ed. 2014, 53, 9780–9783. [Google Scholar] [CrossRef]
- Xie, Z.L.; Xu, H.B.; Geßner, A.; Kumke, M.U.; Priebe, M.; Fromm, K.M.; Taubert, A. A transparent, flexible, ion conductive, and luminescent PMMA ionogel based on a Pt/Eu bimetallic complex and the ionic liquid [Bmim][N(Tf)2]. J. Mater. Chem. 2012, 22, 8110. [Google Scholar] [CrossRef] [Green Version]
- Delahaye, E.; Göbel, R.; Löbbicke, R.; Guillot, R.; Sieber, C.; Taubert, A. Silica ionogels for proton transport. J. Mater. Chem. 2012, 22, 17140. [Google Scholar] [CrossRef] [Green Version]
- Neouze, M.A.; Le Bideau, J.; Leroux, F.; Vioux, A. A route to heat resistant solid membranes with performances of liquid electrolytes. Chem. Commun. 2005, 1082–1084. [Google Scholar] [CrossRef]
- Néouze, M.A.; Le Bideau, J.; Gaveau, P.; Bellayer, S.; Vioux, A. Ionogels, new materials arising from the confinement of ionic liquids within silica-derived networks. Chem. Mater. 2006, 18, 3931–3936. [Google Scholar] [CrossRef]
- Le Bideau, J.; Viau, L.; Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, H.; Li, L.; Chen, R.; Guo, S. Ionogel electrolytes for high-performance lithium batteries: A review. Adv. Energy Mater. 2018, 8, 1702675. [Google Scholar] [CrossRef]
- Visentin, A.F.; Panzer, M.J. Poly(ethylene glycol) diacrylate-supported ionogels with consistent capacitive behavior and tunable elastic response. ACS Appl. Mater. Interfaces 2012, 4, 2836–2839. [Google Scholar] [CrossRef] [PubMed]
- Thiemann, S.; Sachnov, S.J.; Pettersson, F.; Bollström, R.; Österbacka, R.; Wasserscheid, P.; Zaumseil, J. Cellulose-based ionogels for paper electronics. Adv. Funct. Mater. 2014, 24, 625–634. [Google Scholar] [CrossRef]
- Terasawa, N. High-performance transparent actuator made from poly(dimethylsiloxane)/ionic liquid gel. Sens. Actuat. B Chem. 2018, 257, 815–819. [Google Scholar] [CrossRef]
- Debecker, D.P.; Mutin, P.H. Non-hydrolytic sol–gel routes to heterogeneous catalysts. Chem. Soc. Rev. 2012, 41, 3624–3650. [Google Scholar] [CrossRef]
- Wang, Y.M.; Ulrich, V.; Donnelly, G.F.; Lorenzini, F.; Marr, A.C.; Marr, P.C. A Recyclable acidic ionic liquid gel catalyst for dehydration: Comparison with an analogous SILP catalyst. ACS Sustain. Chem. Eng. 2015, 3, 792–796. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Lee, A.S.; Lee, J.C.; Hong, S.M.; Hwang, S.S.; Koo, C.M. Hybrid ionogel electrolytes for high temperature lithium batteries. J. Mater. Chem. A 2015, 3, 2226–2233. [Google Scholar] [CrossRef]
- Tan, G.; Wu, F.; Zhan, C.; Wang, J.; Mu, D.; Lu, J.; Amine, K. Solid-state li-ion batteries using fast, stable, glassy nanocomposite electrolytes for good safety and long cycle-life. Nano Lett. 2016, 16, 1960–1968. [Google Scholar] [CrossRef]
- Yang, F.; Wu, D.; Luo, Z.; Tan, B.; Xie, Z. Hybrid organic-inorganic dyeionogels: Reversibly pH-responsive materials based dye-ionic liquids with improved structural stability and flexibility. Sens. Actuat. B Chem. 2017, 249, 486–492. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, D.; Li, Z.; Su, G. Gel polymer electrolytes prepared by in situ polymerization of vinyl monomers in room-temperature ionic liquids. React. Funct. Polym. 2006, 66, 1141–1148. [Google Scholar] [CrossRef]
- Gayet, F.; Viau, L.; Leroux, F.; Mabille, F.; Monge, S.; Robin, J.J.; Vioux, A. Unique combination of mechanical strength, thermal stability, and high ion conduction in PMMA−silica nanocomposites containing high loadings of ionic liquid. Chem. Mater. 2009, 21, 5575–5577. [Google Scholar] [CrossRef]
- Horowitz, A.I.; Westerman, K.; Panzer, M.J. Formulation influence on the sol–gel formation of silica-supported ionogels. J. Sol Gel Sci. Technol. 2016, 78, 34–39. [Google Scholar] [CrossRef]
- Susan, M.A.; Kaneko, T.; Noda, A.; Watanabe, M. Ion gels prepared by in situ radical polymerization of vinyl monomers in an ionic liquid and their characterization as polymer electrolytes. J. Am. Chem. Soc. 2005, 127, 4976–4983. [Google Scholar] [CrossRef] [PubMed]
- Guyomard-Lack, A.; Abusleme, J.; Soudan, P.; Lestriez, B.; Guyomard, D.; Bideau, J.L. Hybrid silica–polymer ionogel solid electrolyte with tunable properties. Adv. Energy Mater. 2014, 4, 1301570. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, S.; Zheng, R.; Zhang, D.; Zhang, H. Silica-based ionogel electrolyte with porous flower-like structure enables safer lithium ion battery. Appl. Surf. Sci. 2019, 485, 119–127. [Google Scholar] [CrossRef]
- Song, H.; Luo, Z.; Zhao, H.; Luo, S.; Wu, X.; Gao, J.; Wang, Z. High tensile strength and high ionic conductivity bionanocomposite ionogels prepared by gelation of cellulose/ionic liquid solutions with nano-silica. RSC Adv. 2013, 3, 11665–11675. [Google Scholar] [CrossRef]
- Horowitz, A.I.; Panzer, M.J. High-performance, mechanically compliant silica-based ionogels for electrical energy storage applications. J. Mater. Chem. 2012, 22, 16534. [Google Scholar] [CrossRef]
- Kato, T.; Okazaki, A.; Hayase, S. Latent gel electrolyte precursors for quasi-solid dye sensitized solar cells. Chem. Commun. 2005, 363–365. [Google Scholar] [CrossRef]
- Shimano, S.; Zhou, H.; Honma, I. Preparation of nanohybrid solid-state electrolytes with liquidlike mobilities by solidifying ionic liquids with silica particles. Chem. Mater. 2007, 19, 5216–5221. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, J.; Chang, L.; Zhang, X.; Liu, H.; Jiang, L. Preparation of high-performance ionogels with excellent transparency, good mechanical strength, and high conductivity. Adv. Mater. 2017, 29, 1704253. [Google Scholar] [CrossRef]
- Zhang, B.; Xue, Y.; Xue, Z.; Li, Z.; Hao, J. A green synthesis of nanosheet-constructed Pd particles in an ionic liquid and their superior electrocatalytic performance. ChemPhysChem 2015, 16, 3865–3870. [Google Scholar] [CrossRef]
- Taubert, A.; Löbbicke, R.; Kirchner, B.; Leroux, F. First examples of organosilica-based ionogels: Synthesis and electrochemical behavior. Beilstein J. Nanotechnol. 2017, 8, 736–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Samples | TEOS/MTMS/MMA Feed Mass Ratio | IL (g) | CD (mg) | QY (%) | Tensile strain | Compressive Strain | TG Decomposition (°C) |
|---|---|---|---|---|---|---|---|
| A1 | 1:1:2 | 0 | 2 | 36% | 1.582 | 0.515 | 177.2 |
| A2 | 1:1:4 | 0 | 2 | 32% | 1.788 | 0.619 | 177.5 |
| A3 | 1:1:6 | 0 | 2 | 30% | 1.476 | 0.557 | 177.5 |
| A4 | 1:1:8 | 0 | 2 | 29% | 1.496 | 0.213 | 178 |
| A5 | 1:1:4 | 0.5 | 2 | 67% | 2.884 | 0.698 | 99 |
| A6 | 1:1:4 | 1.0 | 2 | 69% | 1.474 | 0.840 | 162 |
| A7 | 1:1:4 | 1.5 | 2 | 45% | 0.715 | 0.747 | 189 |
| A8 | 1:1:4 | 2.0 | 2 | 48% | 0.340 | 0.544 | 192 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, L.; Liu, Y.; Wu, D.; Wei, Q.-H.; Taubert, A.; Xie, Z. Luminescent Ionogels with Excellent Transparency, High Mechanical Strength, and High Conductivity. Nanomaterials 2020, 10, 2521. https://doi.org/10.3390/nano10122521
Tao L, Liu Y, Wu D, Wei Q-H, Taubert A, Xie Z. Luminescent Ionogels with Excellent Transparency, High Mechanical Strength, and High Conductivity. Nanomaterials. 2020; 10(12):2521. https://doi.org/10.3390/nano10122521
Chicago/Turabian StyleTao, Lumi, Yuchuan Liu, Dan Wu, Qiao-Hua Wei, Andreas Taubert, and Zailai Xie. 2020. "Luminescent Ionogels with Excellent Transparency, High Mechanical Strength, and High Conductivity" Nanomaterials 10, no. 12: 2521. https://doi.org/10.3390/nano10122521
APA StyleTao, L., Liu, Y., Wu, D., Wei, Q. -H., Taubert, A., & Xie, Z. (2020). Luminescent Ionogels with Excellent Transparency, High Mechanical Strength, and High Conductivity. Nanomaterials, 10(12), 2521. https://doi.org/10.3390/nano10122521